Establishment of potent TCR-T cells specific for cisplatin-resistance related tumor-associated antigen, CLSPN using codon-optimization
- PMID: 39539024
- PMCID: PMC11572277
- DOI: 10.1080/21645515.2024.2414542
Establishment of potent TCR-T cells specific for cisplatin-resistance related tumor-associated antigen, CLSPN using codon-optimization
Abstract
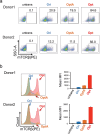
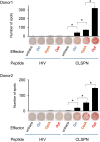
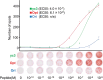
Adoptive T cell therapy, using T cell receptor-engineered T (TCR-T) cells and chimeric antigen receptor T (CAR-T) cells, is a potent immunotherapy option. Bladder cancer is a prevalent urological malignancy, particularly in cases of muscle invasion and metastasis, for which systemic therapy is crucial. Immunotherapy utilizing immune checkpoint blockade has been approved for bladder cancer treatment. The antitumor effect of an immune checkpoint blockade based on cytotoxic T cells (CTLs) and the patient's immune status is essential. The chemotherapeutic drug cisplatin (CDDP) is a key drug in bladder cancer treatment. However, it has been shown to suppress T cells, making combination therapy with CDDP and immunotherapy difficult. To address this, we developed TCR-T cells specific for bladder cancer cells. In previous studies, we found that the tumor-associated antigen CLSPN is overexpressed in CDDP-resistant bladder cancer cells and that the antigenic peptide HLA-A*02:01/CLSPN1254-1262, encoded by CLSPN, could be targeted by a CTL clone. The TCR was cloned from the HLA-A*02:01/CLSPN1254-1262 specific CTL clone yc3. We also designed a codon-optimized TCR sequence using GeneArt® GeneOptimizer® (Opt TCR) and compared the TCR-T cells using the original TCR sequence (Ori TCR-T cells) and the codon-optimized TCR sequence (Opt TCR-T cells). Opt TCR-T cells exhibited higher TCR transduction efficiency, higher TCR expression levels, higher avidity, and greater cytotoxicity than did Ori TCR-T cells. These results suggest that HLA-A*02:01/CLSPN1254-1262 specific Opt TCR-T cells are promising candidates for CDDP combination therapy.
Keywords: CLSPN; TCR-T; Urothelial carcinoma; cisplatin resistance; immunotherapy.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Sternberg CN, Yagoda A, Scher HI, Watson RC, Herr HW, Morse MJ, Sogani PC, Vaughan ED, Bander N, Weiselberg LR. M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium. J Urol. 1988;139(3):461–469. doi:10.1016/S0022-5347(17)42494-3. - DOI - PubMed
-
- von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A, Lippert CM, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–3677. doi:10.1200/JCO.2000.18.17.3068. - DOI - PubMed
-
- von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–4608. doi:10.1200/JCO.2005.07.757. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
