A randomized clinical trial of the impact of melatonin on influenza vaccine: Outcomes from the melatonin and vaccine response immunity and chronobiology study (MAVRICS)
- PMID: 39539030
- PMCID: PMC11572083
- DOI: 10.1080/21645515.2024.2419742
A randomized clinical trial of the impact of melatonin on influenza vaccine: Outcomes from the melatonin and vaccine response immunity and chronobiology study (MAVRICS)
Abstract
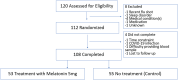
Vaccine immunogenicity is affected by a variety of factors. Melatonin has been reported to affect immune responses to vaccines and infection. This was a randomized open-label trial - in which adults scheduled to receive the influenza vaccine were randomized to 5 mg melatonin or control to evaluate the effect of post-vaccination melatonin on humoral (hemagglutination-inhibition assays, HAI) and cellular (FluoroSpot) vaccine-specific cytokine responses 14-21 days post-vaccination. A total of 108 participants (melatonin treatment group: 53; control group: 55) completed the study. The groups were similar in baseline characteristics, including sleep as measured by the Pittsburgh Sleep Quality Index. Seroconversion rates or geometric mean fold rises (GMFR) in HAI titers did not vary by treatment group. There were also no statistically significant differences between pre- and post-vaccination levels of interferon gamma (IFN-γ) or granzyme B (GzB) by treatment; however, there was a significantly higher fold rise in the double secretor (IFN-γ + GzB) peripheral blood mononuclear cells for influenza vaccine in subjects taking daily melatonin (GMFR 1.7; 95% CI 1.3, 2.3) compared to those who did not (GMFR 0.9; 95% CI 0.7, 1.1) (p < .001). Daily melatonin for 14 days post-influenza vaccination significantly increased the cellular co-expression of IFN-γ + GzB; however, there were no other differences in the cellular or humoral responses. Future studies of the potential utility of melatonin for enhancing vaccine response with larger sample sizes may help elucidate candidate mechanisms for these limited effects, including any interactions with the circadian system.
Keywords: Vaccine immunogenicity; cellular immunity; humoral immunity; influenza vaccine; melatonin; vaccine adjuvant.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- CDC . Past Seasons’ Vaccine Effectiveness Estimates; [accessed 2024 May 17]. https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html.
-
- Spiegel K, Rey AE, Cheylus A, Ayling K, Benedict C, Lange T, Prather AA, Taylor DJ, Irwin MR, Van Cauter E, et al. A meta-analysis of the associations between insufficient sleep duration and antibody response to vaccination. Curr Biol. 2023;33(5):998–1005.e2. doi:10.1016/j.cub.2023.02.017. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical