Postprandial Sleep in Short-Sleeping Mexican Cavefish
- PMID: 39539086
- PMCID: PMC11579814
- DOI: 10.1002/jez.2880
Postprandial Sleep in Short-Sleeping Mexican Cavefish
Abstract
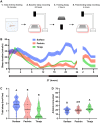
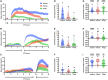
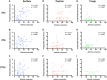
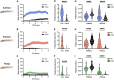
Interactions between sleep and feeding behaviors are critical for adaptive fitness. Diverse species suppress sleep when food is scarce to increase the time spent foraging. Postprandial sleep, an increase in sleep time following a feeding event, has been documented in vertebrate and invertebrate animals. While interactions between sleep and feeding appear to be highly conserved, the evolution of postprandial sleep in response to changes in food availability remains poorly understood. Multiple populations of the Mexican cavefish, Astyanax mexicanus, have independently evolved sleep loss and increased food consumption compared to surface-dwelling fish of the same species, providing the opportunity to investigate the evolution of interactions between sleep and feeding. Here, we investigate the effects of feeding on sleep in larval and adult surface fish, and in two parallelly evolved cave populations of A. mexicanus. Larval surface and cave populations of A. mexicanus increase sleep immediately following a meal, providing the first evidence of postprandial sleep in a fish model. The amount of sleep was not correlated to meal size and occurred independently of feeding time. In contrast to larvae, postprandial sleep was not detected in adult surface or cavefish, which can survive for months without food. Together, these findings reveal that postprandial sleep is present in multiple short-sleeping populations of cavefish, suggesting sleep-feeding interactions are retained despite the evolution of sleep loss. These findings raise the possibility that postprandial sleep is critical for energy conservation and survival in larvae that are highly sensitive to food deprivation.
Keywords: cavefish; circadian; evolution; feeding; sleep.
© 2024 The Author(s). Journal of Experimental Zoology Part A: Ecological and Integrative Physiology published by Wiley Periodicals LLC.
Figures

Update of
-
Postprandial sleep in short-sleeping Mexican cavefish.bioRxiv [Preprint]. 2024 Jul 5:2024.07.03.602003. doi: 10.1101/2024.07.03.602003. bioRxiv. 2024. Update in: J Exp Zool A Ecol Integr Physiol. 2024 Dec;341(10):1084-1096. doi: 10.1002/jez.2880. PMID: 39005273 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
- This work was supported by NIH Grants NIH 1R01GM127872 to S.E.M., Nicolas Rohner, and Alex C. Keene; R24 OD030214 to Wes Warren, Nicolas Rohner, and Alex C. Keene; R21 NS122166 to Alex C. Keene and Johanna E. Kowalko; 1DP2AG071466-01 to Nicolas Rohner; and NSF Grant IOS 2202359 to Johanna E. Kowalko and S.E.M.
- DP2 AG071466/AG/NIA NIH HHS/United States
- R24 OD030214/OD/NIH HHS/United States
- R21 NS122166/NS/NINDS NIH HHS/United States
- R01 GM127872/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

