Tixagevimab-cilgavimab for preventing breakthrough COVID-19 in dialysis patients: a prospective study
- PMID: 39539359
- PMCID: PMC11558061
- DOI: 10.1093/ckj/sfae309
Tixagevimab-cilgavimab for preventing breakthrough COVID-19 in dialysis patients: a prospective study
Abstract
Background: The effectiveness of tixagevimab-cilgavimab as pre-exposure prophylaxis (PrEP) against breakthrough coronavirus disease 2019 (COVID-19) in dialysis patients remains uncertain due to limited data.
Methods: In this multicenter prospective study, we enrolled vaccinated dialysis patients and divided them into two groups: a tixagevimab-cilgavimab group (received a 150 mg/150 mg intramuscular dose of tixagevimab-cilgavimab) and a control group (age-matched patients not receiving tixagevimab-cilgavimab). The primary outcome was the breakthrough COVID-19 rate at 6 months, whereas secondary outcomes included COVID-19-related hospitalization, intensive care unit admission, endotracheal intubation and mortality. The safety of tixagevimab-cilgavimab was assessed.
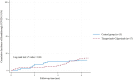
Results: Two hundred participants were enrolled, with equal numbers in each group (n = 100 each). Baseline characteristics were comparable between groups, except for a higher number of COVID-19 vaccine doses in the tixagevimab-cilgavimab group [median (IQR) 4 (3-5) vs. 3 (3-4); P = .01]. At 6 months, the breakthrough COVID-19 rates were comparable between the tixagevimab-cilgavimab (17%) and control (15%) groups (P = .66). However, the median (IQR) time to diagnosis of breakthrough infections tended to be longer in the tixagevimab-cilgavimab group [4.49 (2.81-4.98) vs 1.96 (1.65-2.91) months; P = .08]. Tixagevimab-cilgavimab significantly reduced COVID-19-related hospitalization rates (5.9% vs 40.0%; P = .02) among participants with breakthrough infections. All tixagevimab-cilgavimab-related adverse events were mild.
Conclusion: The use of tixagevimab-cilgavimab as PrEP in vaccinated dialysis patients during the Omicron surge did not prevent breakthrough infections but significantly reduced COVID-19-related hospitalizations. Further research should prioritize alternative strategies.
Keywords: breakthrough COVID-19; dialysis; prophylaxis; safety; tixagevimab–cilgavimab.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
None declared.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

