Structure-function relationship of dynorphin B variants using naturally occurring amino acid substitutions
- PMID: 39539623
- PMCID: PMC11557314
- DOI: 10.3389/fphar.2024.1484730
Structure-function relationship of dynorphin B variants using naturally occurring amino acid substitutions
Abstract
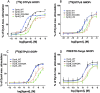
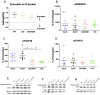
Dynorphins (Dyn) represent the subset of endogenous opioid peptides with the highest binding affinity to kappa opioid receptors (KOPrs). Activation of the G-protein-coupled pathway of KOPrs has strong anticonvulsant effects. Dyn also bind to mu (MOPrs) and delta opioid receptors (DOPrs) with lower affinity and can activate the β-arrestin pathway. To fully exploit the therapeutic potential of dynorphins and reduce potential unwanted effects, increased selectivity for KOPrs combined with reduced activation of the mTOR complex would be favorable. Therefore, we investigated a series of dynorphin B (DynB) variants, substituted in one or two positions with naturally occurring amino acids for differential opioid receptor activation, applying competitive radio binding assays, GTPγS assays, PRESTO-Tango, and Western blotting on single-opioid receptor-expressing cells. Seven DynB derivatives displayed at least 10-fold increased selectivity for KOPrs over either MOPrs or DOPrs. The highest selectivity for KOPrs over MOPrs was obtained with DynB_G3M/Q8H, and the highest selectivity for KOPrs over DOPrs was obtained with DynB_L5S. Increased selectivity for KOPr over MOPr and DOPr was based on a loss of affinity or potency at MOPr and DOPr rather than a higher affinity or potency at KOPr. This suggests that the investigated amino acid exchanges in positions 3, 5, and 8 are of higher importance for binding and activation of MOPr or DOPr than of KOPr. In tests for signal transduction using the GTPγS assay, none of the DynB derivatives displayed increased potency. The three tested variants with substitutions of glycine to methionine in position 3 displayed reduced efficacy and are, therefore, considered partial agonists. The two most promising activating candidates were further investigated for functional selectivity between the G-protein and the β-arrestin pathway, as well as for activation of mTOR. No difference was detected in the respective read-outs, compared to wild-type DynB. Our data indicate that the assessment of affinity to KOPr alone is not sufficient to predict either potency or efficacy of peptidergic agonists on KOPr. Further assessment of downstream pathways is required to allow more reliable predictions of in vivo effects.
Keywords: G-protein-coupled receptors; epilepsy; functional selectivity; opioid receptors; selectivity.
Copyright © 2024 Zangrandi, Fogli, Mutti, Staritzbichler, Most, Hildebrand, Heilbronn and Schwarzer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Electrophysiological analysis of paraventricular thalamic neurons co-expressing kappa and mu opioid receptors.Neuropharmacology. 2025 Jul 1;272:110407. doi: 10.1016/j.neuropharm.2025.110407. Epub 2025 Mar 10. Neuropharmacology. 2025. PMID: 40074169
-
Effects of modifications of residues in position 3 of dynorphin A(1-11)-NH2 on kappa receptor selectivity and potency.J Med Chem. 1996 Jun 21;39(13):2456-60. doi: 10.1021/jm950655o. J Med Chem. 1996. PMID: 8691442
-
Nicotine and endogenous opioids: neurochemical and pharmacological evidence.Neuropharmacology. 2011 Jun;60(7-8):1209-20. doi: 10.1016/j.neuropharm.2010.11.010. Epub 2010 Nov 22. Neuropharmacology. 2011. PMID: 21108953 Review.
-
Big dynorphin as a putative endogenous ligand for the kappa-opioid receptor.J Neurochem. 2006 Apr;97(1):292-301. doi: 10.1111/j.1471-4159.2006.03732.x. Epub 2006 Mar 3. J Neurochem. 2006. PMID: 16515546
-
The Opioid System in Temporal Lobe Epilepsy: Functional Role and Therapeutic Potential.Front Mol Neurosci. 2017 Aug 7;10:245. doi: 10.3389/fnmol.2017.00245. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28824375 Free PMC article. Review.
References
-
- Aldrich J. V., Zheng Q. I., Murray T. F. (2001). Dynorphin a analogs containing a conformationally constrained phenylalanine derivative in position 4: reversal of preferred stereochemistry for opioid receptor affinity and discrimination of kappa vs. Delta receptors. Chirality 13, 125–129. 10.1002/1520-636X(2001)13:3<125::AID-CHIR1008>3.0.CO;2-S - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

