Quality-adjusted time without symptoms or toxicity analysis of haploidentical-related donor vs. identical sibling donor hematopoietic stem cell transplantation in acute myeloid leukemia
- PMID: 39539811
- PMCID: PMC11555205
- DOI: 10.21147/j.issn.1000-9604.2024.05.06
Quality-adjusted time without symptoms or toxicity analysis of haploidentical-related donor vs. identical sibling donor hematopoietic stem cell transplantation in acute myeloid leukemia
Abstract
Objective: We aimed to compare the quality-adjusted time without symptoms or toxicity (Q-TWiST) in acute myeloid leukemia (AML) patients who received haploidentical-related donor (HID) and identical sibling donor (ISD) hematopoietic stem cell transplantation (HSCT).
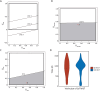
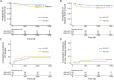
Methods: Five clinical health states were defined: toxicity (TOX), acute graft-versus-host disease (GVHD), chronic GVHD (cGVHD), time without symptoms and toxicity (TWiST) and relapse (REL). The equation used in this study was as follows: Q-TWiST=UTOX × TOX + UTWiST × TWiST + UREL × REL + UaGVHD × aGVHD + UcGVHD × cGVHD.
Results: A total of 239 AML patients were enrolled. We established a mathematical model, i.e., Q-TWiST HID HSCT > Q-TWiST ISD HSCT, to explore the range of utility coefficients satisfying the inequality. Based on the raw data, the utility coefficient is equivalent to the following inequality: [Formula: see text][Formula: see text]. The model showed that when [Formula: see text], [Formula: see text], and [Formula: see text] were within the range of 0-1, as well as when [Formula: see text] was within the range of 0-0.569, the inequality Q-TWiST HID HSCT > Q-TWiST ISD HSCT was valid. According to the results of the ChiCTR1800016972 study, the median coefficients of TOX, acute GVHD (aGVHD), and cGVHD were 0.56 (0.41-0.76), 0.56 (0.47-0.72), and 0.54 (0.37-0.79), respectively. We selected a series of specific examples of the coefficients, i.e., [Formula: see text]=0.5, [Formula: see text]=0.05, [Formula: see text]=0.5, and [Formula: see text]=0.5. The Q-TWiST values of ISD and HID HSCT were 896 and 900 d, respectively (P=0.470).
Conclusions: We first observed that Q-TWiST was comparable between AML patients receiving HID HSCT and those receiving ISD HSCT.
Keywords: Quality-adjusted time without symptoms or toxicity; acute myeloid leukemia; allogeneic hematopoietic stem cell transplantation; haploidentical.
Copyright ©2024 Chinese Journal of Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures








Similar articles
-
Unmanipulated haplo-identical donor transplantation compared with identical sibling donor had better anti-leukemia effect for refractory/relapsed acute myeloid leukemia not in remission status.Ann Hematol. 2020 Dec;99(12):2911-2925. doi: 10.1007/s00277-020-04283-0. Epub 2020 Oct 1. Ann Hematol. 2020. PMID: 33000361
-
Comparable outcomes among unmanipulated haploidentical, matched unrelated, and matched sibling donors in BU-based myeloablative hematopoietic stem cell transplantation for intermediate and adverse risk acute myeloid leukemia in complete remission: a single-center study.Ann Hematol. 2021 Jun;100(6):1579-1591. doi: 10.1007/s00277-020-04355-1. Epub 2020 Nov 24. Ann Hematol. 2021. PMID: 33236196
-
Haploidentical- versus identical-sibling transplant for high-risk pediatric AML: A multi-center study.Cancer Commun (Lond). 2020 Mar;40(2-3):93-104. doi: 10.1002/cac2.12014. Epub 2020 Mar 16. Cancer Commun (Lond). 2020. PMID: 32175698 Free PMC article.
-
Effects of different types of allogeneic hematopoietic stem cell transplantation donors on Philadelphia chromosome-positive acute lymphoblastic leukemia during the tyrosine kinase inhibitor era: A systematic review and meta-analysis.Hematol Oncol Stem Cell Ther. 2023 Apr 4;16(3):197-208. doi: 10.1016/j.hemonc.2021.09.007. Hematol Oncol Stem Cell Ther. 2023. PMID: 34743893
-
Haploidentical Transplantation with Post-Transplant Cyclophosphamide versus Unrelated Donor Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis.Biol Blood Marrow Transplant. 2019 Dec;25(12):2422-2430. doi: 10.1016/j.bbmt.2019.07.028. Epub 2019 Aug 3. Biol Blood Marrow Transplant. 2019. PMID: 31386903
Cited by
-
Comparison of plasma sterilizer and conventional laminar flow room in allogeneic hematopoietic stem cell transplant recipients.Cell Transplant. 2025 Jan-Dec;34:9636897251335722. doi: 10.1177/09636897251335722. Epub 2025 Apr 30. Cell Transplant. 2025. PMID: 40305491 Free PMC article.
-
Venetoclax and azacitidine compared with intensive chemotherapy for adverse-risk acute myeloid leukemia patients receiving allogeneic hematopoietic stem cell transplantation in first complete remission: A multicenter study of TROPHY group.Chin J Cancer Res. 2025 Jun 30;37(3):417-431. doi: 10.21147/j.issn.1000-9604.2025.03.10. Chin J Cancer Res. 2025. PMID: 40642494 Free PMC article.
References
-
- Shouval R, Fein JA, Labopin M, et al Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: a European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol. 2019;6:e573–84. doi: 10.1016/S2352-3026(19)30158-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources
