Unveiling the dynamics of circulating tumor cells in colorectal cancer: from biology to clinical applications
- PMID: 39539964
- PMCID: PMC11557528
- DOI: 10.3389/fcell.2024.1498032
Unveiling the dynamics of circulating tumor cells in colorectal cancer: from biology to clinical applications
Abstract
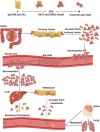
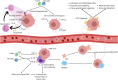
This review delves into the pivotal role of circulating tumor cells (CTCs) in colorectal cancer (CRC) metastasis, focusing on their biological properties, interactions with the immune system, advanced detection techniques, and clinical implications. We explored how metastasis-competent CTCs evade immune surveillance and proliferate, utilizing cutting-edge detection and isolation technologies, such as microfluidic devices and immunological assays, to enhance sensitivity and specificity. The review highlights the significant impact of CTC interactions with immune cells on tumor progression and patient outcomes. It discusses the application of these findings in clinical settings, including non-invasive liquid biopsies for early diagnosis, prognosis, and treatment monitoring. Despite advancements, challenges remain, such as the need for standardized methods to consistently capture and analyze CTCs. Addressing these challenges through further molecular and cellular research on CTCs could lead to improved interventions and outcomes for CRC patients, underscoring the importance of unraveling the complex dynamics of CTCs in cancer progression.
Keywords: cancer cell biology; circulating tumor cells; colorectal cancer; liquid biopsy; metastasis.
Copyright © 2024 Dompé, Chojnowska, Ramlau, Nowicki, Alix-Panabières and Budna-Tukan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Aktar S., Hamid F. B., Gamage S. M. K., Cheng T., Parkneshan N., Lu C. T., et al. (2023a). Gene expression analysis of immune regulatory genes in circulating tumour cells and peripheral blood mononuclear cells in patients with colorectal carcinoma. Int. J. Mol. Sci. 24, 5051. 10.3390/ijms24055051 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

