Results of the prospective EORTC Children Leukemia Group study 58081 in precursor B- and T-cell acute lymphoblastic leukemia
- PMID: 39540141
- PMCID: PMC11558101
- DOI: 10.1002/hem3.70025
Results of the prospective EORTC Children Leukemia Group study 58081 in precursor B- and T-cell acute lymphoblastic leukemia
Abstract
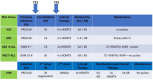
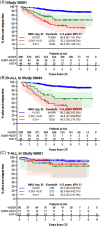
Here, we report the results of the prospective cohort study EORTC-CLG 58081 and compare them to the control arm of the randomized phase 3 trial EORTC-CLG 58951, on which treatment recommendations were built. In both studies, patients aged 1-18 years with BCR::ABL1 negative acute lymphoblastic leukemia of the B-lineage (B-ALL) or T-lineage (T-ALL) were treated using a BFM backbone without cranial irradiation. Similarly to the control arm of 58951, prednisolone (PRED) 60 mg/m2/day was used for induction therapy, but a few modifications were made. Dexamethasone (DXM) was used in average-risk 2 (AR2) T-ALL and B-ALL during induction, 10 and 6 mg/m2/day, respectively. Leucovorin rescue was delayed to 42 h instead of 36 h after initiation of high-dose methotrexate, and a postconsolidation MRD time point was added to stratify patients. Between 2011 and 2017, 835 patients were prospectively enrolled in the 58081 study. Overall, the 5-year event-free survival (EFS) was 84.8% versus 83.6% (hazard ratio [HR], 0.96 [95% confidence interval [CI]: 0.76-1.21]) for 58081 versus 58951 considered as a control group, respectively, 84.3% versus 84.9% (HR, 1.06 [99% CI: 0.75-1.49]) in B-ALL but 87.3% versus 76.6% (HR, 0.59 [99% CI: 0.28-1.24]) in T-ALL. The comparison between the two studies regarding EFS differed by risk group (p = 0.012). The HR was 2.15 (99% CI: 0.67-6.85) for very low-risk but 0.34 (99% CI: 0.13-0.89) for AR2. The particularly favorable results observed in the T-ALLs and AR2 subgroups suggest the benefit of using DXM in specific patient groups and highlight the importance of risk stratification.
© 2024 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
All authors declare no conflict of interest.
Figures




References
-
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371(9617):1030‐1043. - PubMed
-
- Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B‐cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP‐BFM ALL 2000 study. Blood. 2010;115(16):3206‐3214. - PubMed
-
- Cavé H, van der Werff ten Bosch J, Suciu S, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. N Engl J Med. 1998;339(9):591‐598. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
