Ixazomib, pomalidomide and dexamethasone in relapsed or refractory multiple myeloma characterized with highrisk cytogenetics: the IFM 2014-01 study
- PMID: 39540213
- PMCID: PMC11873707
- DOI: 10.3324/haematol.2024.285916
Ixazomib, pomalidomide and dexamethasone in relapsed or refractory multiple myeloma characterized with highrisk cytogenetics: the IFM 2014-01 study
Figures
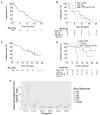
References
-
- D’agostino M, Cairns DA, Lahuerta JJ, et al. . Second Revision of the International Staging System (R2-ISS) for overall survival in multiple myeloma: a European Myeloma Network (EMN) report within the HARMONY Project. J Clin Oncol. 2022;40(29):3406-3418. - PubMed
-
- Leleu X, Karlin L, Macro M, et al. . Pomalidomide plus low-dose dexamethasone in multiple myeloma with deletion 17p and/or translocation (4;14): IFM 2010-02 trial results. Blood. 2015;125(9):1411-1417. - PubMed
-
- Vincent Rajkumar S. Multiple myeloma: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol. 2013;88(3):226-235. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

