Organoid-based precision medicine in pancreatic cancer
- PMID: 39540683
- PMCID: PMC11866314
- DOI: 10.1002/ueg2.12701
Organoid-based precision medicine in pancreatic cancer
Abstract
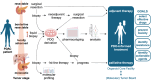
Pancreatic ductal adenocarcinoma (PDAC) ranks among the leading causes of cancer-related deaths worldwide. Despite advances in precision oncology in other malignancies, treatment of PDAC still largely relies on conventional chemotherapy. Given the dismal prognosis and heterogeneity in PDAC, there is an urgent need for personalized therapeutic strategies to improve treatment response. Organoids, generated from patients' tumor tissue, have emerged as a powerful tool in cancer research. These three-dimensional models faithfully recapitulate the morphological and genetic features of the parental tumor and retain patient-specific heterogeneity. This review summarizes existing precision oncology approaches in PDAC, explores current applications and limitations of organoid cultures in personalized medicine, details preclinical studies correlating in vitro organoid prediction and patient treatment response, and provides an overview of ongoing organoid-based clinical trials.
Keywords: PDAC; drug response prediction; oncology; pancreatic ductal adenocarcinoma; patient‐derived organoids; personalized therapy; pharmacotyping; precision oncology; treatment response.
© 2024 The Author(s). United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
T. Ettrich reports personal fees from MSD, Roche, Sanofi, BMS, AstraZeneca, Merck Serono, Pierre Fabre, Servier, Lilly, Ipsen, Daiichi Sankyo, AbbVie, Takeda, Amgen and grants from Servier, Lilly (Inst) outside of the submitted work. T. Seufferlein reports grants and personal fees from Celgene and Sanofi, personal fees from Amgen, AstraZeneca, Bayer, the Falk Foundation, Lilly, Merck‐Serono, Merck, Pierre Fabre, Roche, Servier, and Shire, and grants from Boehringer Ingelheim outside the submitted work. A. Kleger reports personal fees from the Falk foundation and Amgen outside the submitted work. No disclosures were reported by the other authors.
Figures

References
-
- NCCN Guidelines (Online) . National comprehensive cancer network. 2024. Available from: http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
-
- Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC‐4): a multicentre, open‐label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–1024. 10.1016/s0140-6736(16)32409-6 - DOI - PubMed
-
- Burris HA3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. 10.1200/jco.1997.15.6.2403 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

