Safety, outcomes, and pharmacokinetics of isavuconazole as a treatment for invasive fungal diseases in pediatric patients: a non-comparative phase 2 trial
- PMID: 39540734
- PMCID: PMC11642194
- DOI: 10.1128/aac.00484-24
Safety, outcomes, and pharmacokinetics of isavuconazole as a treatment for invasive fungal diseases in pediatric patients: a non-comparative phase 2 trial
Abstract
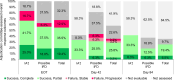
Invasive aspergillosis (IA) and mucormycosis (IM) cause significant morbidity and mortality in immunocompromised and/or hospitalized patients. Isavuconazonium sulfate, a prodrug of the antifungal triazole isavuconazole, has been approved for treatment of IA and IM in adults; and was recently approved in children. This study describes the outcomes, safety, and pharmacokinetics of isavuconazole for the treatment of proven, probable, or possible IA or IM in children. In this phase 2, open-label, non-comparative study, patients aged 1 to <18 years with at least possible invasive mold disease were enrolled across 10 centers in the US, Spain, and Belgium from 2019 to 2022. Patients received 10 mg/kg isavuconazonium sulfate daily (maximum 372 mg; equivalent to 5.4 mg/kg or 200 mg isavuconazole) for up to 84 (IA) or 180 days (IM). Outcomes included rates of all-cause case fatality, overall response, treatment-emergent adverse events (TEAEs), and pharmacokinetics. Of 31 patients enrolled, 61.3% were 1-<12 years old; 58.1% had underlying hematologic malignancies. The successful overall response rate at the end of treatment was 54.8%. Day 42 all-cause case fatality was 6.5%; 93.5% experienced TEAEs, and two patients discontinued treatment due to drug-related TEAEs. Dosing at 10 mg/kg (maximum dose: 372 mg) met the pre-defined exposure threshold of above the 25th percentile for the area under the concentration-time curve (≥60 mg·h/L). Simulated doses of 15 mg/kg improved drug exposures in patients aged 1-<3 years. Isavuconazole was well tolerated in children, with exposure consistent with adult studies. Successful response was documented in 54.8% of patients.CLINICAL TRIALSThis study is registered at ClinicalTrials.gov as NCT03816176.
Keywords: invasive fungal diseases; isavuconazole; pediatrics.
Conflict of interest statement
The authors declare a conflict of interest (see Acknowledgments).
Figures


References
-
- Olivier-Gougenheim L, Rama N, Dupont D, Saultier P, Leverger G, AbouChahla W, Paillard C, Gandemer V, Theron A, Freycon C, et al. . 2021. Invasive fungal infections in immunocompromised children: novel insight following a national study. J Pediatr 236:204–210. doi:10.1016/j.jpeds.2021.05.016 - DOI - PubMed
-
- Patterson TF, Thompson GR III, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young J-AH, Bennett JE. 2016. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis 63:e1–e60. doi:10.1093/cid/ciw326 - DOI - PMC - PubMed
-
- Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Flörl C, Lewis RE, Munoz P, Verweij PE, et al. . 2018. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 24 Suppl 1:e1–e38. doi:10.1016/j.cmi.2018.01.002 - DOI - PubMed
-
- Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, Hoenigl M, Jensen HE, Lagrou K, Lewis RE, et al. . 2019. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 19:e405–e421. doi:10.1016/S1473-3099(19)30312-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

