Whole-blood model reveals granulocytes as key sites of dengue virus propagation, expanding understanding of disease pathogenesis
- PMID: 39540772
- PMCID: PMC11633123
- DOI: 10.1128/mbio.01505-24
Whole-blood model reveals granulocytes as key sites of dengue virus propagation, expanding understanding of disease pathogenesis
Abstract
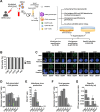
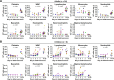
Dengue virus (DENV) infection poses a significant global health threat, yet our understanding of its immunopathogenesis remains incomplete due to limitations of existing models. Here, we establish an in vitro whole-blood model using hirudin, an anticoagulant that preserves complement activity and cellular interactions, to study DENV infection. Our model reveals the susceptibility of all major leukocyte populations to DENV infection, with monocytes and granulocytes demonstrating high permissiveness and production of infectious virus progeny. Notably, granulocytes emerge as previously unrecognized targets of DENV infection, highlighting the importance of studying viral tropism within a physiologically relevant context. We also observed efficient DENV binding to B cells, but limited production of infectious virus, suggesting a potential role in viral sequestration or immune dysregulation. Interestingly, both NK and T cells, while less permissive, were also found to be susceptible to DENV infection. Our ex vivo analysis of whole blood from DENV-infected patients confirms the susceptibility of granulocytes, monocytes, B cells, natural killer cells, and T cells to infection, further validating the clinical relevance of our model. Additionally, we observed dynamic changes in circulating blood cell populations during acute dengue, potentially reflecting both direct virus-mediated effects and immune responses. This whole-blood model offers a valuable tool for investigating the complex interplay between DENV and host factors, facilitating a deeper understanding of dengue pathogenesis and ultimately contributing to the development of novel therapeutic strategies.IMPORTANCEDengue virus (DENV) infection is a significant global health threat, with increasing incidence in endemic regions and expanding geographic range due to factors like global warming. Current models for studying DENV pathogenesis often lack the complexity of the human immune system, hindering the development of effective therapies and vaccines. To address this, we have established the first in vitro whole-blood model using hirudin, preserving critical immune components and cellular interactions. This model reveals granulocytes as previously unrecognized targets of productive DENV infection, challenging existing paradigms of viral tropism. Our ex vivo analysis of patient blood samples confirms the clinical relevance of this finding and validates our model's utility. This unique model offers a powerful platform for future studies to dissect the complex interactions between DENV and the host immune system, including the roles of different leukocyte populations, ultimately informing the development of novel therapeutic strategies to combat this devastating disease.
Keywords: dengue target cells; dengue virus; granulocytes; in vitro whole-blood infection model; neutrophils.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature New Biol 496:504–507. doi:10.1038/nature12060 - DOI - PMC - PubMed
-
- Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, Hay SI, Bedi N, Bensenor IM, Castañeda-Orjuela CA, Chuang T-W, Gibney KB, Memish ZA, Rafay A, Ukwaja KN, Yonemoto N, Murray CJL. 2016. The global burden of dengue: an analysis from the global burden of disease study 2013. Lancet Infect Dis 16:712–723. doi:10.1016/S1473-3099(16)00026-8 - DOI - PMC - PubMed
MeSH terms
Grants and funding
- PHD/0026/2557/Royal Golden Jubilee (RGJ) Ph.D. Programme (RGJ-Ph.D. programme)
- FF-027/2566/National Research and Innovation Fund (NSRF)
- R016234004/MU | Faculty of Medicine Siriraj Hospital, Mahidol University (Siriraj Hospital)
- R35-GM136352-01/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- Research Excellence Development (RED)/MU | Faculty of Medicine Siriraj Hospital, Mahidol University (Siriraj Hospital)
LinkOut - more resources
Full Text Sources
Medical
