A Phase III Randomized Trial of Integrated Genomics and Avatar Models for Personalized Treatment of Pancreatic Cancer: The AVATAR Trial
- PMID: 39540844
- PMCID: PMC11739777
- DOI: 10.1158/1078-0432.CCR-23-4026
A Phase III Randomized Trial of Integrated Genomics and Avatar Models for Personalized Treatment of Pancreatic Cancer: The AVATAR Trial
Abstract
Purpose: Pancreatic ductal adenocarcinoma (PDAC) has limited treatment options. We compared the efficacy of comprehensive precision medicine against that of the conventional treatment in PDAC.
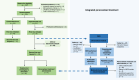
Patients and methods: We report a phase III trial of advanced PDAC in which patients were randomized (1:2) to a conventional treatment treated at physician's discretion (arm A) or to precision medicine (arm B). Subjects randomized to arm B underwent a tumor biopsy for whole-exome sequencing and to generate avatar mouse models and patient-derived organoids for phenotypic drug screening, with final treatment recommended by the molecular tumor board. The primary objective was median overall survival (OS).
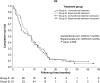
Results: A total of 137 patients were enrolled with 125 randomized, 44 to arm A and 81 to arm B. Whole-exome sequencing was performed in 80.3% (65/81) patients of arm B, with potentially actionable mutations detected in 21.5% (14/65). Experimental models were generated in 16/81 patients (19.8%). Second-line treatment was administered to 39 patients in the experimental arm, but only four (10.2%) received personalized treatment, whereas 35 could not receive matched therapy because of rapid clinical deterioration, delays in obtaining study results, or the absence of actionable targets. The median OS was 8.7 and 8.6 months (P = 0.849) and the median progression-free survival was 3.8 and 4.3 months (P = 0.563) for the conventional and experimental arms, respectively. Notably, the four patients who received personalized treatment had a median OS of 19.3 months.
Conclusions: Personalized medicine was challenging to implement in most patients with PDAC, limiting the interpretation of intention-to-treat analysis. Survival was improved in the subset of patients who did receive matched therapy.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
F. Sarno reports grants and personal fees from the European Commission during the conduct of the study. R. Pazo-Cid reports other support from Astellas, Ipsen, Roche, BeiGene, Bristol Myers Squibb, Servier, Eli Lilly and Company, and AstraZeneca and personal fees from F. Hoffmann-La Roche Ltd., Eisai, Bristol Myers Squibb, Amgen, Astellas, Roche, and Eli Lilly and Company outside the submitted work. R. Garcia-Carbonero reports personal fees from AAA-Novartis, Advanz Pharma, Astellas, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Esteve, GSK, HUTCHMED, Ipsen, Merck, Midatech Pharma, MSD, PharmaMar, Pierre Fabre, Roche, and Servier and grants from Pfizer, Bristol Myers Squibb, and MSD during the conduct of the study. J. Feliu reports personal fees from Roche, AstraZeneca, and Viatris and grants from Amgen during the conduct of the study. C. Guillen-Ponce reports other support from QED Therapeutics, AstraZeneca, Boston, Erytech, Ipsen, Panbela Therapeutics, OncoSil Medical, Theriva, Roche, and GE Healthcare outside the submitted work. C. Guerra reports grants from the Spanish government, i.e., Acción Estratégica en Salud 2019, del subprograma de proyectos de Investigación en Salud during the conduct of the study, as well as a patent [patent application EP18382555 (M. Barbacid, C. Guerra M.T. Blasco, and C. Navas, 2018)] related to Combined Therapy against Cancer and a patent for Solicitud de patente europea No. 23382078.6 pending. Y. Duran reports grants and personal fees from the European Commission during the conduct of the study. C. Alonso reports grants from the European Research Council during the conduct of the study. P. Lapunzina reports personal fees from Roche Institute-Spain, Novo Nordisk, and Pfizer during the conduct of the study, as well as personal fees from Roche Institute-Spain, Novo Nordisk, and Pfizer during the conduct of the study, related to scientific advice and lectures on Personalized Medicine and Genetics. B. Bockorny reports research funding from Agenus Inc. and NanoView Biosciences; travel expenses from Erytech Pharma; and advisory board and consulting fees from Blueprint Medicines, BioLineRx, and Enlivex. M. Hidalgo reports other support from Bristol Myers Squibb and Nelum, personal fees and other support from Champions Oncology, InxMed, and OncoMatrix, and personal fees from MiNKi and Peaches outside the submitted work, as well as being a member of the Bristol Myers Squibb board of directors. No disclosures were reported by the other authors.
Figures


References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48. - PubMed
-
- Sohal DPS, Kennedy EB, Cinar P, Conroy T, Copur MS, Crane CH, et al. . Metastatic pancreatic cancer: ASCO guideline update. J Clin Oncol 2020;38:3217–30. - PubMed
-
- Taieb J, Prager GW, Melisi D, Westphalen CB, D’Esquermes N, Ferreras A, et al. . First-line and second-line treatment of patients with metastatic pancreatic adenocarcinoma in routine clinical practice across Europe: a retrospective, observational chart review study. ESMO Open 2020;5:e000587. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

