Hepatic arterial infusion chemotherapy combined with systemic therapy sequentially or simultaneously for advanced hepatocellular carcinoma
- PMID: 39540963
- PMCID: PMC11564491
- DOI: 10.1007/s00262-024-03872-6
Hepatic arterial infusion chemotherapy combined with systemic therapy sequentially or simultaneously for advanced hepatocellular carcinoma
Abstract
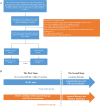
Background and aims: The goal of this study was to compare the efficacy and safety of hepatic arterial infusion chemotherapy (HAIC) combined with targeted therapy and PD-(L)1 blockade (triple therapy), either sequentially (SE) or simultaneously (SI), in the treatment of Barcelona Clinic Liver Cancer (BCLC) stage C hepatocellular carcinoma (HCC).
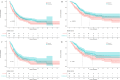
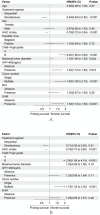
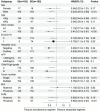
Approach and results: From January 1, 2018, to June 1, 2022, 575 patients with BCLC stage C HCC who underwent SE or SI triple therapy were retrospectively enrolled. Propensity score matching (PSM; 1:1) was performed to eliminate possible confounder imbalances across cohorts. We used the Kaplan-Meier method and a log-rank test to compare the overall survival (OS) and progression-free survival (PFS) rates between the SI and SE groups. The tumor response and the incidence of adverse events (AEs) were reported. After PSM, 182 patients in each of the two groups were matched. The median OS in the SI group was significantly longer than that in the SE group (28.8 vs. 16.1 months; P = 0.002), and the median PFS was significantly improved in the SI versus SE group (9.6 vs. 7.0 months; P = 0.01). The objective response rate based on the mRECIST was higher in the SI group (58% vs. 37%; P < 0.001). The total incidences of grade 3-4 AEs were 111/182 (60.9%) and 128/182 (70.3%) in the SE and SI groups, respectively. No grade 5 AEs were reported in either group.
Conclusions: Simultaneous HAIC plus targeted therapy and PD-(L)1 blockade significantly improved outcomes compared to the sequential regimen in patients with BCLC stage C HCC, with no unexpected AEs.
Clinical relevance statement: The patients who received hepatic arterial infusion chemotherapy combined with targeted therapy and PD-(L)1 blockade simultaneously have a better prognosis than those who received it sequentially.
Keywords: Hepatic arterial infusion chemotherapy; Hepatocellular carcinoma; PD-(L)1 blockade; Sequentially and simultaneously; Targeted therapy.
© 2024. The Author(s).
Conflict of interest statement
Figures
Similar articles
-
Efficiency and safety of hepatic arterial infusion chemotherapy (HAIC) combined with anti-PD1 therapy versus HAIC monotherapy for advanced hepatocellular carcinoma: A multicenter propensity score matching analysis.Cancer Med. 2024 Jan;13(1):e6836. doi: 10.1002/cam4.6836. Epub 2024 Jan 9. Cancer Med. 2024. PMID: 38196277 Free PMC article.
-
Combining immune checkpoint inhibitors and molecular-targeted agents with hepatic arterial infusion chemotherapy for hepatocellular carcinoma with inferior vena cava and/or right atrium tumor thrombus.Hepatol Int. 2025 Jun;19(3):560-575. doi: 10.1007/s12072-025-10777-8. Epub 2025 Feb 11. Hepatol Int. 2025. PMID: 39934618
-
Conversion study of hepatocellular carcinoma using HAIC combined with lenvatinib and PD-1/L1 immunotherapy under the guidance of BCLC staging.Front Immunol. 2025 Jun 2;16:1596864. doi: 10.3389/fimmu.2025.1596864. eCollection 2025. Front Immunol. 2025. PMID: 40529364 Free PMC article.
-
Hepatic arterial infusion chemotherapy enhances the efficacy of lenvatinib and PD-1 inhibitors for advanced hepatocellular carcinoma: A meta-analysis and trial sequential analysis.Eur J Surg Oncol. 2025 Mar;51(3):109573. doi: 10.1016/j.ejso.2025.109573. Epub 2025 Jan 6. Eur J Surg Oncol. 2025. PMID: 39793379 Review.
-
Systematic review of hepatic arterial infusion chemotherapy versus sorafenib in patients with hepatocellular carcinoma with portal vein tumor thrombosis.J Gastroenterol Hepatol. 2020 Aug;35(8):1277-1287. doi: 10.1111/jgh.15010. Epub 2020 Mar 3. J Gastroenterol Hepatol. 2020. PMID: 32052876
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249 - PubMed
-
- El-Serag HB, Rudolph KL (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132(7):2557–2576 - PubMed
-
- Chen LT, Martinelli E, Cheng AL, Pentheroudakis G, Qin S, Bhattacharyya GS, Ikeda M, Lim HY, Ho GF, Choo SP et al (2020) Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO. MOS and SSO Ann Oncol 31(3):334–351 - PubMed
-
- Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL et al (2009) Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 10(1):35–43 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

