Toripalimab Plus Chemotherapy as a First-Line Therapy for Extensive-Stage Small Cell Lung Cancer: The Phase 3 EXTENTORCH Randomized Clinical Trial
- PMID: 39541202
- PMCID: PMC11565370
- DOI: 10.1001/jamaoncol.2024.5019
Toripalimab Plus Chemotherapy as a First-Line Therapy for Extensive-Stage Small Cell Lung Cancer: The Phase 3 EXTENTORCH Randomized Clinical Trial
Abstract
Importance: Patients with extensive-stage small cell lung cancer (ES-SCLC) have poor prognoses and unmet medical needs.
Objective: To evaluate the efficacy and safety of toripalimab plus etoposide and platinum-based chemotherapy (EP) vs placebo plus EP as a first-line treatment for patients with ES-SCLC.
Design, setting, and participants: This multicenter, double-blind, placebo-controlled phase 3 randomized clinical trial (EXTENTORCH study) enrolled patients from September 26, 2019, to May 20, 2021, and was conducted at 49 sites in China. Eligible patients had histologically or cytologically confirmed ES-SCLC without previous systemic antitumor therapy for ES-SCLC. Data were analyzed between May 6, 2023, and June 1, 2024.
Interventions: Patients were randomized (1:1) to receive toripalimab, 240 mg, or placebo plus EP every 3 weeks for up to 4 to 6 cycles, followed by maintenance with toripalimab or placebo until disease progression, intolerable toxic effects, or up to 2 years of treatment.
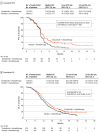
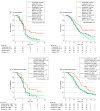
Main outcomes and measures: The primary end points were investigator-assessed progression-free survival (PFS) and overall survival (OS). Whole-exome sequencing results identified correlative biomarkers for clinical efficacy.
Results: Among 595 screened patients, 442 eligible patients were randomized (median [range] age, 63 [30-77] years; 366 [82.8%] male); 223 patients were randomized to toripalimab plus EP, and 219 to placebo plus EP. By April 20, 2023, the median (range) survival follow-up was 13.7 (0.0-42.7) months. Compared with placebo, toripalimab improved investigator-assessed PFS (hazard ratio [HR], 0.67 [95% CI, 0.54-0.82]; P < .001), and significantly reduced the risk of death (HR, 0.80 [95% CI, 0.65-0.98]; P = .03). The median OS was 14.6 (95% CI, 12.9-16.6) months in the toripalimab group vs 13.3 (95% CI, 11.8-14.4) months in the placebo group. Whole-exome sequencing results from 300 patients identified low intratumor heterogeneity, HLA-A11+ HLA-B62- haplotype, wild-type KMT2D and COL4A4, or sequence variations in CTNNA2 or SCN4A correlated with favorable PFS and OS in the toripalimab group. No new safety signals were observed. Grade 3 or higher treatment-emergent adverse event incidence was similar between the toripalimab and placebo safety set groups (199 of 222 patients [89.6%] vs 193 of 216 patients [89.4%], respectively).
Conclusions and relevance: In this phase 3 randomized clinical trial, adding toripalimab to first-line chemotherapy demonstrated significant improvements in PFS and OS for patients with ES-SCLC. The treatment exhibited an acceptable safety profile, supporting this combination regimen as a new treatment option for patients with ES-SCLC.
Trial registration: ClinicalTrials.gov Identifier: NCT04012606.
Conflict of interest statement
Figures

Comment on
-
Toripalimab for Extensive-Stage Small Cell Lung Cancer.JAMA Oncol. 2025 Jan 1;11(1):26-27. doi: 10.1001/jamaoncol.2024.4958. JAMA Oncol. 2025. PMID: 39541197 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

