Nirsevimab Effectiveness Against Medically Attended Respiratory Syncytial Virus Illness and Hospitalization Among Alaska Native Children - Yukon-Kuskokwim Delta Region, Alaska, October 2023-June 2024
- PMID: 39541241
- PMCID: PMC11576050
- DOI: 10.15585/mmwr.mm7345a1
Nirsevimab Effectiveness Against Medically Attended Respiratory Syncytial Virus Illness and Hospitalization Among Alaska Native Children - Yukon-Kuskokwim Delta Region, Alaska, October 2023-June 2024
Abstract
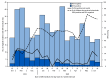
Respiratory syncytial virus (RSV) is a leading cause of hospitalization among young children. Historically, American Indian and Alaska Native (AI/AN) children have experienced high rates of RSV-associated hospitalization. In August 2023, a preventive monoclonal antibody (nirsevimab) was recommended for all infants aged <8 months (born during or entering their first RSV season) and for children aged 8-19 months (entering their second RSV season) who have increased risk for severe RSV illness, including all AI/AN children. This evaluation in Alaska's Yukon-Kuskokwim Delta region estimated nirsevimab effectiveness among AI/AN children in their first or second RSV seasons during 2023-2024. Among 472 children with medically attended acute respiratory illness (ARI), 48% overall had received nirsevimab ≥7 days earlier (median = 91 days before the ARI-related visit). For children in their first RSV season (292), nirsevimab effectiveness was 76% (95% CI = 42%-90%) against medically attended RSV illness and 89% (95% CI = 32%-98%) against RSV hospitalization. For children in their second RSV season (180), effectiveness against medically attended RSV illness was 88% (95% CI = 48%-97%). Nirsevimab is effective for preventing severe RSV illness among infants entering their first RSV season and children entering their second season with increased risk for severe RSV, including all AI/AN children.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. James W. Keck reports grant support from the National Institutes of Health (NIH) for the Alaska Native Center for Health Research Project and for the Wastewater Assessment for Coronavirus in Kentucky: Implementing Enhanced Surveillance Technology; from the National Science Foundation for the Pandemic Environmental Surveillance Center for Assessing Pathogen Emergency Project; from NIH for the COVID-19 infection and diabetes incidence in Native American People project; from NIH for the Neqkiuryaraq – The Art of Preparing Food Project; from Greenwall Foundation for the developing stakeholder-engaged ethical guidance for public health wastewater surveillance project service; and as safety officer and chair of the Data Safety Monitoring Board for National Institute of Diabetes and Digestive and Kidney Diseases-funded study: Enhancing the Diabetes Prevention Program. No other potential conflicts of interest were disclosed.
Figures
References
-
- Li Y, Wang X, Blau DM, et al.; Respiratory Virus Global Epidemiology Network; RESCEU investigators. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022;399:2047–64. 10.1016/S0140-6736(22)00478-0 - DOI - PMC - PubMed
-
- Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:920–5. 10.15585/mmwr.mm7234a4 - DOI - PMC - PubMed
-
- Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus–associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:1115–22. 10.15585/mmwr.mm7241e1 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

