Sequence modeling and design from molecular to genome scale with Evo
- PMID: 39541441
- PMCID: PMC12057570
- DOI: 10.1126/science.ado9336
Sequence modeling and design from molecular to genome scale with Evo
Abstract
The genome is a sequence that encodes the DNA, RNA, and proteins that orchestrate an organism's function. We present Evo, a long-context genomic foundation model with a frontier architecture trained on millions of prokaryotic and phage genomes, and report scaling laws on DNA to complement observations in language and vision. Evo generalizes across DNA, RNA, and proteins, enabling zero-shot function prediction competitive with domain-specific language models and the generation of functional CRISPR-Cas and transposon systems, representing the first examples of protein-RNA and protein-DNA codesign with a language model. Evo also learns how small mutations affect whole-organism fitness and generates megabase-scale sequences with plausible genomic architecture. These prediction and generation capabilities span molecular to genomic scales of complexity, advancing our understanding and control of biology.
Conflict of interest statement
Figures
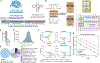



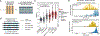

Comment in
-
Learning the language of DNA.Science. 2024 Nov 15;386(6723):729-730. doi: 10.1126/science.adt3007. Epub 2024 Nov 14. Science. 2024. PMID: 39541478
References
-
- Dobzhansky T, Genetics and the Origin of Species (Columbia Univ. Press, 1951).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

