Isolation of psychedelic-responsive neurons underlying anxiolytic behavioral states
- PMID: 39541450
- PMCID: PMC11588385
- DOI: 10.1126/science.adl0666
Isolation of psychedelic-responsive neurons underlying anxiolytic behavioral states
Abstract
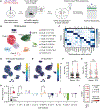
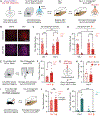
Psychedelics hold promise as alternate treatments for neuropsychiatric disorders. However, the neural mechanisms by which they drive adaptive behavioral effects remain unclear. We isolated the specific neurons modulated by a psychedelic to determine their role in driving behavior. Using a light- and calcium-dependent activity integrator, we genetically tagged psychedelic-responsive neurons in the medial prefrontal cortex (mPFC) of mice. Single-nucleus RNA sequencing revealed that the psychedelic drove network-level activation of multiple cell types beyond just those expressing 5-hydroxytryptamine 2A receptors. We labeled psychedelic-responsive mPFC neurons with an excitatory channelrhodopsin to enable their targeted manipulation. We found that reactivation of these cells recapitulated the anxiolytic effects of the psychedelic without driving its hallucinogenic-like effects. These findings reveal essential insight into the cell-type-specific mechanisms underlying psychedelic-induced behavioral states.
Conflict of interest statement
Figures



References
-
- Roth BL, Gumpper RH, Psychedelics as Transformative Therapeutics. Am J Psychiatry 180, 340–347 (2023). - PubMed
-
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D, Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9, 3897–3902 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

