PD-L1 thresholds predict efficacy of immune checkpoint inhibition in first-line treatment of advanced gastroesophageal adenocarcinoma. A systematic review and meta-analysis of seven phase III randomized trials
- PMID: 39541621
- PMCID: PMC11613432
- DOI: 10.1016/j.esmoop.2024.103967
PD-L1 thresholds predict efficacy of immune checkpoint inhibition in first-line treatment of advanced gastroesophageal adenocarcinoma. A systematic review and meta-analysis of seven phase III randomized trials
Abstract
Background: High expression of programmed death-ligand 1 (PD-L1) has been recognized as a marker of improved efficacy of immunotherapy in gastroesophageal adenocarcinoma (GEA); however, the optimal PD-L1 cut-off is still debated. The aim of the present review was to analyze available phase III trials and to identify the appropriate PD-L1 expression cut-off for GEA.
Methods: Phase III trials investigating the efficacy of anti-programmed cell death protein 1 (PD-1) therapies in addition to standard chemotherapy versus standard chemotherapy in the first-line setting were selected. Progression-free survival (PFS), overall survival (OS) and objective response rate (ORR) were the analyzed outcome measures. Pooled treatment effects were assessed in the unselected population and in subpopulations with different levels of PD-L1 expression.
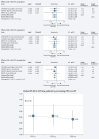
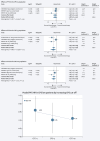
Results: PD-1 blockade efficacy was found to consistently increase in a linear manner with higher combined positive score (CPS) of PD-L1 expression: pooled hazard ratio (HR) for OS and PFS and pooled odds ratio (OR) for ORR of 0.80, 0.75 and 1.51, respectively, in the unselected population versus 0.67, 0.63 and 1.90, respectively, in the CPS ≥10 population (all P values < 0.0001). In the PD-L1-negative population (CPS <1) a significant benefit of anti-PD-1 agents could not be demonstrated in terms of OS and PFS (P = 0.28 and 0.12, respectively), but it was seen in terms of ORR (P = 0.03). PD-1 blockade was effective in the CPS <10 population (P value for pooled OS HR, PFS HR and response OR are all 0.01), while in the CPS <5 population the effect was of borderline significance for OS (P = 0.07) and significant for PFS and ORR (P = 0.02 and 0.03, respectively).
Conclusion: The present meta-analysis confirmed that the benefit of PD-1 blockade in GEA patients is related to PD-L1 CPS, with increased benefit observed for higher CPS cut-offs and no OS benefit in the CPS <1 subset. Overall, data indicate that PD-L1 CPS ≥5 could represent an acceptable cut-off to optimize the risk/benefit ratio of such agents. Our data suggest a potential clinical benefit of immunotherapy in selected patients within the CPS 1-4 population which needs further investigation.
Keywords: PD-L1; gastroesophageal adenocarcinoma; immune checkpoint inhibitors.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Lordick F., Carneiro F., Cascinu S., et al. ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–1020. - PubMed
-
- Formica V., Morelli C., Patrikidou A., et al. A systematic review and meta-analysis of PD-1/PD-L1 inhibitors in specific patient subgroups with advanced gastro-oesophageal junction and gastric adenocarcinoma. Crit Rev Oncol Hematol. 2021;157 - PubMed
-
- Formica V., Morelli C., Patrikidou A., et al. Gastric Inflammatory Prognostic Index (GIPI) in patients with metastatic gastro-esophageal junction/gastric cancer treated with PD-1/PD-L1 immune checkpoint inhibitors. Target Oncol. 2020;15(3):327–336. - PubMed
-
- Shah M.A., Kennedy E.B., Alarcon-Rozas A.E., et al. Immunotherapy and targeted therapy for advanced gastroesophageal cancer: ASCO guideline. J Clin Oncol. 2023;41(7):1470–1491. Erratum in: J Clin Oncol. 2023:JCO2300441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

