Prospect of emerging treatments for hepatitis B virus functional cure
- PMID: 39541952
- PMCID: PMC11925432
- DOI: 10.3350/cmh.2024.0855
Prospect of emerging treatments for hepatitis B virus functional cure
Abstract
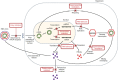
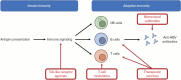
Functional cure, defined as sustained hepatitis B surface antigen (HBsAg) seroclearance with unquantifiable hepatitis B virus (HBV) DNA at 24 weeks off treatment, is a favorable treatment endpoint in chronic hepatitis B (CHB). Nonetheless, functional cure is rarely attained with the current treatment modalities of nucleos(t)ide analogues (NUCs) and pegylated interferon alpha. Multiple novel virus-targeting agents and immunomodulators are under development for HBV with functional cure as the treatment goal. Among virus-targeting agents, antisense oligonucleotides and small-interfering RNAs are the most advanced in the developmental pipeline, and can induce potent and sustainable HBsAg suppression. The other virus-targeting agents have varying effects on HBsAg and HBV DNA, depending on the drug mechanism. In contrast, immunomodulators have modest effects on HBsAg and have limited roles in monotherapy. Multiple combination regimens incorporating RNA interference agents with immunomodulators have been studied through many ongoing clinical trials. These combination strategies demonstrate synergistic effects in inducing functional cure, and will likely be the future direction of development. Despite the promising results, research is warranted to optimize treatment protocols and to establish criteria for NUC withdrawal after novel therapies. Functional cure is now an attainable target in CHB, and the emerging novel therapeutics will revolutionize CHB management.
Keywords: Antiviral agents; Chronic hepatitis B; Hepatitis B surface antigen; Hepatitis B virus; Interferons.
Conflict of interest statement
MF Yuen is an advisory board member and/or received research funding from AbbVie, Arbutus Biopharma, Assembly Biosciences, Bristol Myer Squibb, Dicerna Pharmaceuticals, GlaxoSmithKline, Gilead Sciences, Janssen, Merck Sharp and Dohme, Clear B Therapeutics, Springbank Pharmaceuticals; and received research funding from Arrowhead Pharmaceuticals, Fujirebio Incorporation and Sysmex Corporation. WK Seto received speaker’s fees from AstraZeneca and Echosens, is an advisory board member and received speaker’s fees of Abbott, received research funding from Alexion Pharmaceuticals, Boehringer Ingelheim, Pfizer and Ribo Life Science, and is an advisory board member, received speaker’s fees and researching funding from Gilead Sciences. The remaining authors have no conflict of interests.
Figures
References
-
- World Health Organization (WHO). Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection. WHO web site, < https://www.who.int/publications/i/item/9789240090903>. Accessed 25 Sep 2024.
-
- Ghany MG, Buti M, Lampertico P, Lee HM, 2022 AASLD-EASL HBV-HDV Treatment Endpoints Conference Faculty Guidance on treatment endpoints and study design for clinical trials aiming to achieve cure in chronic hepatitis B and D: report from the 2022 AASLD-EASL HBV-HDV Treatment Endpoints Conference. Hepatology. 2023;78:1654–1673. - PubMed
-
- Mak LY, Seto WK, Hui RW, Fung J, Wong DK, Lai CL, et al. Fibrosis evolution in chronic hepatitis B e antigen-negative patients across a 10-year interval. J Viral Hepat. 2019;26:818–827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

