CD39 activities in the treated acupoints contributed to the analgesic mechanism of acupuncture on arthritis rats
- PMID: 39542981
- PMCID: PMC12454817
- DOI: 10.1007/s11302-024-10065-4
CD39 activities in the treated acupoints contributed to the analgesic mechanism of acupuncture on arthritis rats
Abstract
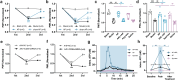
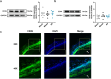
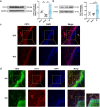
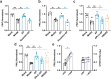
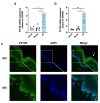
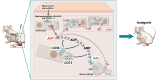
Our previous work had identified that at the acupuncture point (acupoint), acupuncture-induced ATP release was a pivotal event in the initiation of analgesia. We aimed to further elucidate the degradation of ATP by CD39. Acupuncture was administered at Zusanli acupoint on arthritis rats, and pain thresholds of the hindpaws were determined. Pharmacological tools or adeno-associated viruses were administered at the acupoints to interfere with targeting signals. Protein expression was determined with qRT-PCR, WB, or immunofluorescent labeling. Cultured keratinocytes, HaCaT line, were subjected to hypotonic shock to simulate needling stimulation. Extracellular ATP and adenosine levels were quantified using luciferase-luciferin assay and ELISA, respectively. Acupuncture-induced prompt analgesia was impaired by inhibiting CD39 activities to prevent the degradation of ATP to AMP but was mimicked by using CD39 agonists. Acupuncture-induced ATP accumulation exhibited synchronous changes. Similarly, acupuncture analgesia was hindered by suppressing CD73 to prevent the conversion of AMP to adenosine. Furthermore, the acupuncture effect was replicated by agonism at P2Y2Rs but inhibited by antagonism at them. Acupuncture upregulated CD73 and P2Y2Rs but not CD39. Immunofluorescent labeling demonstrated that keratinocytes were a primary site for these proteins. Shallow acupuncture also demonstrated antinociception. In vitro tests showed that hypotonic shock induced HaCaT cells to release ATP and adenosine, which was impaired by suppressing CD39 and CD73, respectively. Finally, agonism at P2Y2Rs promoted ATP release and [Ca2+]i rise. CD39 at the acupoints contributes to the analgesic mechanism of acupuncture. It may facilitate adenosine signaling in conjunction with CD73 or provide an appropriate ATP milieu for P2Y2Rs. Skin tissue may be one of the scenes for these signalings.
Keywords: ATP; Acupuncture analgesia; Acupuncture points; CD39; CD73; P2Y2.
© 2024. The Author(s), under exclusive licence to Springer Nature B.V.
Conflict of interest statement
Declarations. Ethical approval: All experimental protocols were approved by the Animal Care Committee of Shanghai University of Traditional Chinese Medicine (Shanghai, China) (protocol code: PZSHUTCM2212270003). Conflict of interest: The authors declare no competing interests.
Figures





References
-
- Ding G, Zhang D, Huang M, Wang L, Yao W (2012) The function of collagen and mast cells at acupoints. In: Xia Y, Ding G, Wu G (eds) The function of collagen and mast cells at acupoints, Eds. Springer, Springer Location, pp 53–87
-
- Burnstock G (2009) Acupuncture: a novel hypothesis for the involvement of purinergic signalling. Med Hypotheses 73:470–472. 10.1016/j.mehy.2009.05.031 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

