Induction of intercrypt goblet cells upon bacterial infection: a promising therapeutic target to restore the mucosal barrier
- PMID: 39543081
- PMCID: PMC11572077
- DOI: 10.1080/19490976.2024.2426609
Induction of intercrypt goblet cells upon bacterial infection: a promising therapeutic target to restore the mucosal barrier
Abstract
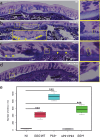
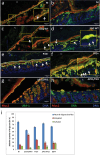
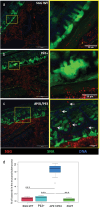
Intestinal mucins play a crucial role in the mucosal barrier, serving as the body's initial defense against microorganisms. However, how the host regulates the secretion and glycosylation of these mucins in response to bacterial invasion remains unclear. Our study demonstrates that when exposed to Streptococcus gallolyticus (SGG), a gut pathobiont, the host mucosa promptly adjusts the behavior of specialized goblet cells (GCs) located in the middle of the crypts. A subset of these cells undergoes a remodeling, becoming intercrypt goblet cells (icGCs), which do not detach from the surface but instead migrate along intercrypt spaces while secreting a mucus impermeable to bacterial pathogens. Significantly, a non-piliated SGG mutant unable to bind to mucus fails to induce icGCs, allowing its translocation through the mucosa and submucosa. Interestingly, a closely related nonpathogenic bacterium, SGM, able to bind to mucus, also triggers the differentiation of GCs into icGCs. This discovery opens new avenues for treating patients with a "leaky gut" as observed in intestinal diseases such as inflammatory bowel diseases and metabolic disorders, but also patients with a history of repeated antibiotic use. Utilizing mucus-adherent probiotics to induce icGCs represents a promising strategy for reinforcing the mucosal barrier.
Keywords: Intercrypt goblet cells; Streptococcus gallolyticus; infection; intestinal mucosal barrier; mucin sialylation; mucus-binding bacteria; pili.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
