Progress and prospects of mRNA-based drugs in pre-clinical and clinical applications
- PMID: 39543114
- PMCID: PMC11564800
- DOI: 10.1038/s41392-024-02002-z
Progress and prospects of mRNA-based drugs in pre-clinical and clinical applications
Abstract
In the last decade, messenger ribonucleic acid (mRNA)-based drugs have gained great interest in both immunotherapy and non-immunogenic applications. This surge in interest can be largely attributed to the demonstration of distinct advantages offered by various mRNA molecules, alongside the rapid advancements in nucleic acid delivery systems. It is noteworthy that the immunogenicity of mRNA drugs presents a double-edged sword. In the context of immunotherapy, extra supplementation of adjuvant is generally required for induction of robust immune responses. Conversely, in non-immunotherapeutic scenarios, immune activation is unwanted considering the host tolerability and high expression demand for mRNA-encoded functional proteins. Herein, mainly focused on the linear non-replicating mRNA, we overview the preclinical and clinical progress and prospects of mRNA medicines encompassing vaccines and other therapeutics. We also highlight the importance of focusing on the host-specific variations, including age, gender, pathological condition, and concurrent medication of individual patient, for maximized efficacy and safety upon mRNA administration. Furthermore, we deliberate on the potential challenges that mRNA drugs may encounter in the realm of disease treatment, the current endeavors of improvement, as well as the application prospects for future advancements. Overall, this review aims to present a comprehensive understanding of mRNA-based therapies while illuminating the prospective development and clinical application of mRNA drugs.
© 2024. The Author(s).
Conflict of interest statement
Figures
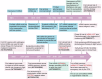
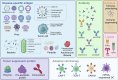
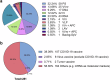
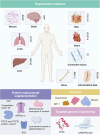
Similar articles
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
Cited by
-
Rational design of lipid nanoparticles for enabling gene therapies.Mol Ther Methods Clin Dev. 2025 Jun 18;33(3):101518. doi: 10.1016/j.omtm.2025.101518. eCollection 2025 Sep 11. Mol Ther Methods Clin Dev. 2025. PMID: 40687376 Free PMC article. Review.
-
Technological breakthroughs and advancements in the application of mRNA vaccines: a comprehensive exploration and future prospects.Front Immunol. 2025 Mar 4;16:1524317. doi: 10.3389/fimmu.2025.1524317. eCollection 2025. Front Immunol. 2025. PMID: 40103818 Free PMC article. Review.
-
The Potential of RNA Therapeutics in Treating Cardiovascular Disease.Drugs. 2025 May;85(5):659-676. doi: 10.1007/s40265-025-02173-1. Epub 2025 Apr 2. Drugs. 2025. PMID: 40175855 Review.
-
Synthesis of 2-Substituted Adenosine Triphosphate Derivatives and their use in Enzymatic Synthesis and Postsynthetic Labelling of RNA.Chembiochem. 2025 Jun 16;26(12):e202500241. doi: 10.1002/cbic.202500241. Epub 2025 May 21. Chembiochem. 2025. PMID: 40317833 Free PMC article.
-
Peptides: potential delivery systems for mRNA.RSC Chem Biol. 2025 Feb 26;6(5):666-677. doi: 10.1039/d4cb00295d. eCollection 2025 May 8. RSC Chem Biol. 2025. PMID: 40071030 Free PMC article. Review.
References
-
- Bonelli, M. et al. Additional heterologous versus homologous booster vaccination in immunosuppressed patients without SARS-CoV-2 antibody seroconversion after primary mRNA vaccination: a randomised controlled trial. Ann. Rheum. Dis.81, 687–694 (2022). - PubMed
-
- Brenner, S., Jacob, F. & Meselson, M. An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature190, 576–581 (1961). - PubMed
-
- Wolff, J. A. et al. Direct gene transfer into mouse muscle in vivo. Science247, 1465–1468 (1990). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

