Structure confirmation, reactivity, bacterial mutagenicity and quantification of 2,2,4-tribromo-5-hydroxycyclopent-4-ene-1,3-dione in drinking water
- PMID: 39543162
- PMCID: PMC11564736
- DOI: 10.1038/s42004-024-01356-3
Structure confirmation, reactivity, bacterial mutagenicity and quantification of 2,2,4-tribromo-5-hydroxycyclopent-4-ene-1,3-dione in drinking water
Abstract
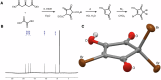
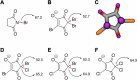
The presence of two new disinfectant by-product (DBP) groups in the UK was recently shown using non-target analysis, halogenated-hydroxycyclopentenediones and halogenated-methanesulfonic acids. In this work, we confirmed the structure of 2,2,4-tribromo-5-hydroxycyclopent-4-ene-1,3-dione (TBHCD), and quantified it together with dibromomethanesulfonic acid at 122 ± 34 and 326 ± 157 ng L-1 on average in London's drinking water, respectively (n = 21). We found TBHCD to be photolabile and unstable in tap water and at alkaline pH. Furthermore, spectral and computational data for TBHCD and three other halogenated-hydroxycyclopentenediones indicated they could act as a source of radicals in water and in the body. Importantly, TBHCD was calculated to have a 14.5 kcal mol-1 lower C-Br bond dissociation enthalpy than the N-Br bond of N-bromosuccinimide, a common radical substitution reagent used in organic synthesis. TBHCD was mutagenic in Salmonella/microsome assays using strains TA98, TA100 and TA102. This work reveals the unique features, activity and toxicity of trihalogenated hydroxycyclopent-4-ene-1,3-diones, prompting a need to more comprehensively assess their risks.
© 2024. The Author(s).
Conflict of interest statement
Figures


Similar articles
-
Comparative mutagenicity of halogenated pyridines in the Salmonella typhimurium/mammalian microsome test.Mutat Res. 1987 Feb;176(2):185-98. doi: 10.1016/0027-5107(87)90049-2. Mutat Res. 1987. PMID: 3543664
-
[The mutagenicity of organic microcontamination in the environment. II. The mutagenicity of volatile organic halogens in the Salmonella microsome test (Ames Test) with regard to the contamination of groundwater and drinking water].Zentralbl Bakteriol Mikrobiol Hyg B Umwelthyg Krankenhaushyg Arbeitshyg Prav Med. 1989 Feb;187(3):230-43. Zentralbl Bakteriol Mikrobiol Hyg B Umwelthyg Krankenhaushyg Arbeitshyg Prav Med. 1989. PMID: 2494817 German.
-
Genotoxic activity of chlorinated butenoic acids in Salmonella typhimurium strains TA98, TA100 and TA104.Mutat Res. 1998 Sep 1;417(1):31-7. doi: 10.1016/s1383-5718(98)00092-8. Mutat Res. 1998. PMID: 9729256
-
Mutation spectra in salmonella of chlorinated, chloraminated, or ozonated drinking water extracts: comparison to MX.Environ Mol Mutagen. 1995;26(4):270-85. doi: 10.1002/em.2850260403. Environ Mol Mutagen. 1995. PMID: 8575416
-
Mutagens in surface waters: a review.Mutat Res. 2004 Nov;567(2-3):109-49. doi: 10.1016/j.mrrev.2004.08.003. Mutat Res. 2004. PMID: 15572284 Review.
References
-
- Wang, Z., Walker, G. W., Muir, D. C. & Nagatani-Yoshida, K. Toward a global understanding of chemical pollution: a first comprehensive analysis of national and regional chemical inventories. Environ. Sci. Technol.54, 2575–2584 (2020). - PubMed
-
- Ciccarelli, D. et al. Bridging knowledge gaps in human chemical exposure via drinking water with non-target screening. Crit. Rev. Environ. Sci. Technol. 1–25 10.1080/10643389.2024.2396690 (2024).
LinkOut - more resources
Full Text Sources
Miscellaneous

