Gut microbes of a high-value marine fish, Snubnose Pompano (Trachinotus blochii) are resilient to therapeutic dosing of oxytetracycline
- PMID: 39543167
- PMCID: PMC11564560
- DOI: 10.1038/s41598-024-75319-y
Gut microbes of a high-value marine fish, Snubnose Pompano (Trachinotus blochii) are resilient to therapeutic dosing of oxytetracycline
Abstract
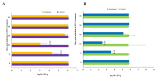
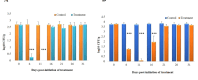
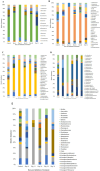
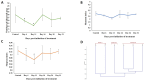
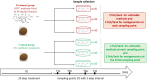
Trachinotus blochii is a high-value tropical mariculture species. The present study evaluated the gut microbial impact of therapeutic exposure (80 mg/day/kg biomass for 10 days) to oxytetracycline, the most common aquaculture antibiotic in T. blochii. The cultivable counts, α-diversity measures of taxonomic and functional metagenomics, microbial dysbiosis (MD) index, and microbial taxon abundances showed the resilience of gut microbiota at 16-26 days of treatment. A significant reduction in bacterial abundance, diversity measures, Firmicutes and Actinobacteria and an increase in γ-Proteobacteria was recorded on the 6th and 11th day of treatment. The increased metagenomic stress signatures, decreased beneficial bacterial abundances, decreased abundance of microbial pathways on energy metabolism, and MD index indicated short-term transient stress during the initial days of therapeutic withdrawal, warranting health management measures. Therapeutic exposure reduced the abundance of fish pathogens, including Vibrio spp., kanamycin and ampicillin-resistant bacteria. Strikingly, oxytetracycline treatment did not increase tetracycline-resistant bacterial counts and the predicted abundance of tetracycline resistance encoding genes in the gut, illustrating that therapeutic application would not pose a risk in the context of antimicrobial resistance in short term. Altogether, the present study provides a foundation for oxytetracycline treatment to develop suitable risk minimization tactics in sustainable aquaculture.
Keywords: AMR; Antibiotic; Gut microbial impact; Microbial dysbiosis; Probiotics.
© 2024. The Author(s).
Conflict of interest statement
Figures


References
-
- The state of world fisheries and aquaculture 2022. Towards blue transformation. FAO. FAO & Rome Italy. (2022). 10.4060/cc0461en (Accessed on 03 Mar 2024).
-
- Naylor, R. L. et al. A 20-year retrospective review of global aquaculture. Nature. 591, 551–563. 10.1038/s41586-021-03308-6 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

