Disruption of circadian rhythm as a potential pathogenesis of nocturia
- PMID: 39543359
- PMCID: PMC12285584
- DOI: 10.1038/s41585-024-00961-0
Disruption of circadian rhythm as a potential pathogenesis of nocturia
Abstract
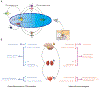
Increasing evidence suggested the multifactorial nature of nocturia, but the true pathogenesis of this condition still remains to be elucidated. Contemporary clinical medications are mostly symptom based, aimed at either reducing nocturnal urine volume or targeting autonomic receptors within the bladder to facilitate urine storage. The day-night switch of the micturition pattern is controlled by circadian clocks located both in the central nervous system and in the peripheral organs. Arousal threshold and secretion of melatonin and vasopressin increase at night-time to achieve high-quality sleep and minimize nocturnal urine production. In response to the increased vasopressin, the kidney reduces the glomerular filtration rate and facilitates the reabsorption of water. Synchronously, in the bladder, circadian oscillation of crucial molecules occurs to reduce afferent sensory input and maintain sufficient bladder capacity during the night sleep period. Thus, nocturia might occur as a result of desynchronization in one or more of these circadian regulatory mechanisms. Disrupted rhythmicity of the central nervous system, kidney and bladder (known as the brain-kidney-bladder circadian axis) contributes to the pathogenesis of nocturia. Novel insights into the chronobiological nature of nocturia will be crucial to promote a revolutionary shift towards effective therapeutics targeting the realignment of the circadian rhythm.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

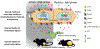
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

