Charge-Stabilized Nanodiscs as a New Class of Lipid Nanoparticles
- PMID: 39543433
- PMCID: PMC11681300
- DOI: 10.1002/adma.202408307
Charge-Stabilized Nanodiscs as a New Class of Lipid Nanoparticles
Abstract
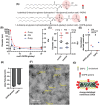
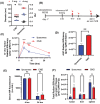
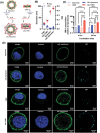
Nanoparticles have the potential to improve disease treatment and diagnosis due to their ability to incorporate drugs, alter pharmacokinetics, and enable tissue targeting. While considerable effort is placed on developing spherical lipid-based nanocarriers, recent evidence suggests that high aspect ratio lipid nanocarriers can exhibit enhanced disease site targeting and altered cellular interactions. However, the assembly of lipid-based nanoparticles into non-spherical morphologies has typically required incorporating additional agents such as synthetic polymers, proteins, lipid-polymer conjugates, or detergents. Here, charged lipid headgroups are used to generate stable discoidal lipid nanoparticles from mixed micelles, which are termed charge-stabilized nanodiscs (CNDs). The ability to generate CNDs in buffers with physiological ionic strength is restricted to lipids with more than one anionic group, whereas monovalent lipids only generate small nanoliposomal assemblies. In mice, the smaller size and anisotropic shape of CNDs promote higher accumulation in subcutaneous tumors than spherical liposomes. Further, the surface chemistry of CNDs can be modified via layer-by-layer (LbL) assembly to improve their tumor-targeting properties over state-of-the-art LbL-liposomes when tested using a metastatic model of ovarian cancer. The application of charge-mediated anisotropy in lipid-based assemblies can aid in the future design of biomaterials and cell-membrane mimetic structures.
Keywords: anisotropy; layer‐by‐layer; lipid nanoparticles; nanodiscs; self‐assembly; tumor targeting.
© 2024 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
I.S.P., P.T.H., and D.J.I. are inventors on a provisional patent filed by the Massachusetts Institute of Technology related to this work.
Figures


References
-
- Durymanov M., Kamaletdinova T., Lehmann S. E., Reineke J., J. Controlled Rel. 2017, 261, 10. - PubMed
-
- Albanese A., Tang P. S., Chan W. C. W., Annu. Rev. Biomed. Eng. 2012, 14, 1. - PubMed
-
- Zhu X., Vo C., Taylor M., Smith B. R., Mater. Horiz. 2019, 6, 1094.
-
- Ding J., Chen J., Gao L., Jiang Z., Zhang Y., Li M., Xiao Q., Lee S. S., Chen X., Nano Today 2019, 29, 100800.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

