Highly prevalent geriatric medications and their effect on β-amyloid fibril formation
- PMID: 39543530
- PMCID: PMC11562802
- DOI: 10.1186/s12883-024-03930-7
Highly prevalent geriatric medications and their effect on β-amyloid fibril formation
Abstract
Background: The unprecedented increase in the older population and ever-increasing incidence of dementia are leading to a "silver tsunami" in upcoming decades. To combat multimorbidity and maintain daily activities, elderly people face a high prevalence of polypharmacy. However, how these medications affect dementia-related pathology, such as Alzheimer's β-amyloid (Aβ) fibrils formation, remains unknown. In the present study, we aimed to analyze the medication profiles of Alzheimer's disease (AD; n = 124), mild cognitive impairment (MCI; n = 114), and non-demented (ND; n = 228) patients to identify highly prevalent drugs and to determine the effects of those drugs on Aβ fibrils formation.
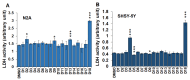
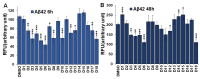
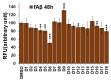
Methods: Study subjects (≥ 65 years) were recruited from an academic geriatric practice that heavily focuses on memory disorders. The disease state was defined based on the score of multiple cognitive assessments. Individual medications for each subject were listed and categorized into 10 major drug classes. Statistical analysis was performed to determine the frequency of individual and collective drug classes, which are expressed as percentages of the respective cohorts. 10 µM monomeric β-amyloid (Aβ) 42 and fibrillar Aβ (fAβ) were incubated for 6-48 h in the presence of 25 µM drugs. fAβ was prepared with a 1:10 ratio of Aβ42 to Aβ40. The amount of Aβ fibrils was monitored using a thioflavin T (Th-T) assay. Neuronal cells (N2A and SHSY-5Y) were treated with 25 µM drugs, and cell death was measured using a lactose dehydrogenase (LDH) assay.
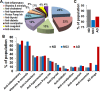
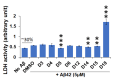
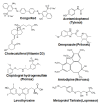
Results: We noticed a high prevalence (82-90%) of polypharmacy and diverse medication profiles including anti-inflammatory (65-77%), vitamin and mineral (64-72%), anti-cholesterol (33-41%), anti-hypersensitive (35-39%), proton pump inhibitor (23-34%), anti-thyroid (9-21%), anti-diabetic (5-13%), anti-constipation (9-11%), anti-coagulant (10-13%), and anti-insomnia (9-20%) drugs in the three cohorts. Our LDH assay with 18 highly prevalent drug components showed toxic effects of Norvasc, Tylenol, Colace, and Plavix on N2A cells, and of vitamin D and Novasc on SH-SY5Y cells. All these drugs except Colace significantly reduced the amount of Aβ fibril when incubated with Aβ42 for a short period (6 h). However, Lipitor, vitamin D, Levothyroxine, Prilosec, Flomax, and Norvasc prominently reduce the amount of fibrils when incubated with monomeric Aβ42 for a longer period (48 h). Furthermore, our disaggregation study with fAβ showed consistent results for cholecalciferol (vitamin D), omeprazole (Prilosec), clopidogrel hydrogensulfate (Flomax), levothyroxine, and amlodipine (Norvasc). The chemical structures of these four efficient molecules contain polyphenol components, a characteristic feature of the structures of polyphenolic inhibitors of Aβ fibrillation.
Conclusion: A higher polypharmacy incidence was observed in an elderly population of 228 ND, 114 MCI, and 124 AD patients. We found that several highly recommended drug components, including vitamin D3, Levothyroxine, Prilosec, Flomax, and Norvasc, efficiently reduce the amount of fibrils formed by monomeric Aβ42 and existing preformed Aβ fibrils in vitro. However, only Levothyroxine was able to prevent Aβ-mediated toxicity to SH-SY5Y cells. Our study suggested that these drugs likely function as polyphenolic inhibitors of Aβ.
Keywords: Alzheimer’s disease; Cytotoxicity; Gariatric medication; Mild cognitive impairment; β-amyoid.
© 2024. The Author(s).
Conflict of interest statement
Figures
References
-
- 2023 Alzheimer’s disease facts and figures. Alzheimers Dement 2023, 19(4):1598–1695. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

