Navigating the intricate in-vivo journey of lipid nanoparticles tailored for the targeted delivery of RNA therapeutics: a quality-by-design approach
- PMID: 39543630
- PMCID: PMC11566655
- DOI: 10.1186/s12951-024-02972-w
Navigating the intricate in-vivo journey of lipid nanoparticles tailored for the targeted delivery of RNA therapeutics: a quality-by-design approach
Abstract
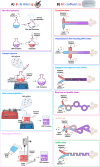
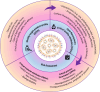
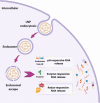
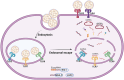
RNA therapeutics, such as mRNA, siRNA, and CRISPR-Cas9, present exciting avenues for treating diverse diseases. However, their potential is commonly hindered by vulnerability to degradation and poor cellular uptake, requiring effective delivery systems. Lipid nanoparticles (LNPs) have emerged as a leading choice for in vivo RNA delivery, offering protection against degradation, enhanced cellular uptake, and facilitation of endosomal escape. However, LNPs encounter numerous challenges for targeted RNA delivery in vivo, demanding advanced particle engineering, surface functionalization with targeting ligands, and a profound comprehension of the biological milieu in which they function. This review explores the structural and physicochemical characteristics of LNPs, in-vivo fate, and customization for RNA therapeutics. We highlight the quality-by-design (QbD) approach for targeted delivery beyond the liver, focusing on biodistribution, immunogenicity, and toxicity. In addition, we explored the current challenges and strategies associated with LNPs for in-vivo RNA delivery, such as ensuring repeated-dose efficacy, safety, and tissue-specific gene delivery. Furthermore, we provide insights into the current clinical applications in various classes of diseases and finally prospects of LNPs in RNA therapeutics.
© 2024. The Author(s).
Conflict of interest statement
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

