Immune dynamics shaping pre-metastatic and metastatic niches in liver metastases: from molecular mechanisms to therapeutic strategies
- PMID: 39543660
- PMCID: PMC11562679
- DOI: 10.1186/s12943-024-02171-z
Immune dynamics shaping pre-metastatic and metastatic niches in liver metastases: from molecular mechanisms to therapeutic strategies
Abstract
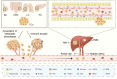
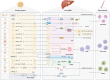
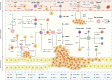
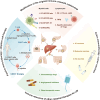
Liver metastases are commonly detected in the advanced stages of various malignant tumors, representing a significant clinical challenge. Throughout the process of liver metastases formation, immune cells play a pivotal role, particularly in the pre-metastatic and metastatic niches within the liver. Immune cells establish extensive and intricate interactions with tumor cells and other components in the liver, collectively promoting and sustaining the growth of liver metastases. Despite the limited efficacy of existing therapeutic modalities against some advanced liver metastases, novel immune-based treatment approaches are continuously being explored and validated. Building on the systematic elucidation of the immunosuppressive characteristics of liver metastases, we explored the potential of novel immunotherapies applicable to patients with liver metastases from multiple dimensions.
Keywords: Immunotherapy; Liver metastases; Tumor immunology; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
Figures


References
-
- Tsilimigras DI, Brodt P, Clavien PA, Muschel RJ, D’Angelica MI, Endo I, Parks RW, Doyle M, de Santibanes E, Pawlik TM. Liver metastases. Nat Rev Dis Primers. 2021;7:27. - PubMed
-
- Ciner AT, Jones K, Muschel RJ, Brodt P. The unique immune microenvironment of liver metastases: challenges and opportunities. Semin Cancer Biol. 2021;71:143–56. - PubMed
-
- Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino T, Martinelli E. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10–32. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

