Endothelial progenitor cell-derived conditioned medium mitigates chronic cerebral ischemic injury through macrophage migration inhibitory factor-activated AKT pathway
- PMID: 39543689
- PMCID: PMC11566597
- DOI: 10.1186/s13287-024-04015-5
Endothelial progenitor cell-derived conditioned medium mitigates chronic cerebral ischemic injury through macrophage migration inhibitory factor-activated AKT pathway
Abstract
Background: Chronic cerebral ischemia (CCI) is a significant health issue characterized by hypoperfusion due to damage or occlusion of the cerebral or carotid arteries. CCI may lead to progressive cognitive impairment that is considered as a prelude to neurodegenerative diseases, including dementia and Alzheimer's disease (AD). Endothelial progenitor cells (EPCs) have been implicated in vascular repair in ischemic cerebrovascular diseases, primarily by differentiating into endothelial cells (ECs) or through paracrine effects. However, the clinical transplantation of stem cell therapies remains limited. In this study, we investigated the effects of EPC-derived conditioned medium (EPC-CM) on the impaired vasculature and neurological function in a rodent model of CCI and the mechanism involved.
Methods: EPC-CM was analyzed by cytokine array to identify key factors involved in angiogenesis and cellular senescence. The effects and mechanism of the candidate factors in the EPC-CM were validated in vitro using oxygen-glucose deprivation (OGD)-injured ECs and EPCs. The therapeutic effects of EPC-CM and the identified key factor were further examined in a rat model of CCI, which was induced by bilateral internal carotid artery ligation (BICAL). EPC-CM was administered via intracisternal injection one week post BICAL. The cerebral microvasculature and neurobehavior of the rats were examined three weeks after BICAL.
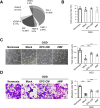
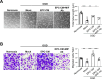
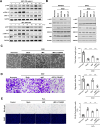
Results: Macrophage migration inhibitory factor (MIF) was identified as a key factor in the EPC-CM. Recombinant MIF protein promoted angiogenesis and prevented senescence in the injured EPCs and ECs. The effect was similar to that of the EPC-CM. These therapeutic effects were diminished when the EPC-CM was co-treated with MIF-specific antibody (Ab). Additionally, the vascular, motor, and cognitive improvements observed in the BICAL rats treated with EPC-CM were abolished by co-treated with MIF Ab. Furthermore, we found MIF promoted angiogenesis and anti-senescence via activating the AKT pathway. Inhibition of the AKT pathway diminished the protective effects of MIF in the in vitro study.
Conclusions: We demonstrated that EPC-CM protected the brain from chronic ischemic injury and promoted functional recovery through MIF-mediated AKT pathway. These findings suggest EPC-CM holds potential as a novel cell-free therapeutic approach for treating CCI through the actions of MIF.
Keywords: AKT pathway; Cerebral ischemia; Endothelial progenitor cell-derived conditioned medium; Macrophage migration inhibitory factor.
© 2024. The Author(s).
Conflict of interest statement
Figures



References
-
- Ciacciarelli A, Sette G, Giubilei F, Orzi F. Chronic cerebral hypoperfusion: an undefined, relevant entity. J Clin Neurosci. 2020;73:8–12. - PubMed
-
- Pappas BA, Davidson CM, Bennett SA, de la Torre JC, Fortin T, Tenniswood MP. Chronic ischemia: memory impairment and neural pathology in the rat. Ann N Y Acad Sci. 1997;826:498–501. - PubMed
-
- Damodaran T, Hassan Z, Navaratnam V, Muzaimi M, Ng G, Müller CP, Liao P, Dringenberg HC. Time course of motor and cognitive functions after chronic cerebral ischemia in rats. Behav Brain Res. 2014;275:252–8. - PubMed
-
- Cechetti F, Pagnussat AS, Worm PV, Elsner VR, Ben J, da Costa MS, Mestriner R, Weis SN, Netto CA. Chronic brain hypoperfusion causes early glial activation and neuronal death, and subsequent long-term memory impairment. Brain Res Bull. 2012;87:109–16. - PubMed
MeSH terms
Substances
Grants and funding
- 108-2314-B-002-086/Ministry of Science and Technology, Taiwan
- 109-2314-B-002-124/Ministry of Science and Technology, Taiwan
- 110-2314-B-002-160-MY2/Ministry of Science and Technology, Taiwan
- 112-2314-B-002-240/Ministry of Science and Technology, Taiwan
- 108-2314-B-002-085-MY2/Ministry of Science and Technology, Taiwan
LinkOut - more resources
Full Text Sources
Miscellaneous

