Age-related STING suppression in macrophages contributes to increased viral load during influenza a virus infection
- PMID: 39543713
- PMCID: PMC11562583
- DOI: 10.1186/s12979-024-00482-9
Age-related STING suppression in macrophages contributes to increased viral load during influenza a virus infection
Abstract
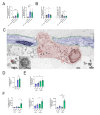
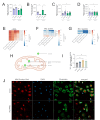
Ageing is a major risk factor that contributes to increased mortality and morbidity rates during influenza A virus (IAV) infections. Macrophages are crucial players in the defense against viral infections and display impaired function during ageing. However, the impact of ageing on macrophage function in response to an IAV infection remains unclear and offers potential insight for underlying mechanisms. In this study, we investigated the immune response of young and aged human monocyte-derived macrophages to two different H1N1 IAV strains. Interestingly, macrophages of aged individuals showed a lower interferon response to IAV infection, resulting in increased viral load. Transcriptomic data revealed a reduced expression of stimulator of interferon genes (STING) in aged macrophages albeit the cGAS-STING pathway was upregulated. Our data clearly indicate the importance of STING signaling for interferon production by applying a THP-1 STING knockout model. Evaluation of mitochondrial function during IAV infection revealed the release of mitochondrial DNA to be the activator of cGAS-STING pathway. The subsequent induction of apoptosis was attenuated in aged macrophages due to decreased STING signaling. Our study provides new insights into molecular mechanisms underlying age-related immune impairment. To our best knowledge, we are the first to discover an age-dependent difference in gene expression of STING on a transcriptional level in human monocyte-derived macrophages possibly leading to a diminished interferon production.
Keywords: Ageing; Influenza A virus; Macrophages; Mitochondria; cGAS-STING pathway.
© 2024. The Author(s).
Conflict of interest statement
Figures



References
-
- Cunha BA. Pneumonia in the elderly. Clin Microbiol Infect. 2001;7:581–8. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

