Tuberculosis Preventive Treatment for Pregnant People With HIV in South Africa: A Modeling Analysis of Clinical Benefits and Risks
- PMID: 39544107
- PMCID: PMC12497950
- DOI: 10.1093/cid/ciae508
Tuberculosis Preventive Treatment for Pregnant People With HIV in South Africa: A Modeling Analysis of Clinical Benefits and Risks
Abstract
Background: Although prior studies of tuberculosis-preventive treatment (TPT) for pregnant people with human immunodeficiency virus (PPWH) report conflicting adverse pregnancy outcome (APO) risks, international guidelines recommend TPT for PPWH.
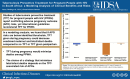
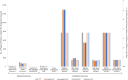
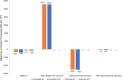
Methods: We used a microsimulation model to evaluate 5 TPT strategies among PPWH receiving antiretroviral therapy in South Africa: No TPT; 6 months of isoniazid (6H) or 3 months of isoniazid-rifapentine (3HP) during pregnancy (Immediate 6H or Immediate 3HP) or post partum (Deferred 6H or Deferred 3HP). The primary outcomes were maternal, fetal/infant, and combined deaths from causes potentially influenced by TPT (maternal tuberculosis, maternal hepatotoxicity, stillbirth, low birth weight [LBW], and infant tuberculosis). Tuberculosis during pregnancy confers 250% and 81% higher modeled risks of stillbirth and LBW, respectively. In lower-risk or higher-risk scenarios, immediate TPT confers 38% lower or 92% higher risks of stillbirth and 16% lower or 35% higher risks of LBW.
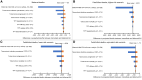
Results: Immediate TPT would minimize deaths among PPWH. When TPT confers higher stillbirth and LBW risks, immediate TPT would produce the most combined maternal and fetal/infant deaths, even with low maternal CD4 cell count and high tuberculosis incidence. If immediate TPT yields a <4% or <20% increase in stillbirth or LBW, immediate TPT would produce fewer combined deaths than deferred TPT (sensitivity analysis range, <2%-22% and <11%-120%, respectively).
Conclusions: If APO risks are below identifiable thresholds, TPT during pregnancy could decrease combined maternal and fetal/infant deaths. Given uncertainty around isoniazid's risks, and the low threshold at which APO risks could outweigh benefits from tuberculosis deaths averted, studies of newer TPT regimens among PPWH are warranted to inform guidelines.
Keywords: HIV; adverse pregnancy outcome; pregnancy; tuberculosis; tuberculosis preventive treatment.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Figures
References
-
- World Health Organization. Tuberculosis (TB). Available at: https://www.who.int/news-room/fact-sheets/detail/tuberculosis. Accessed 9 April 2024.
-
- Rendell NL, Batjargal N, Jadambaa N, Dobler CC. Risk of tuberculosis during pregnancy in Mongolia, a high incidence setting with low HIV prevalence. Int J Tuberc Lung Dis 2016; 20:1615–20. - PubMed
-
- Zenner D, Kruijshaar ME, Andrews N, Abubakar I. Risk of tuberculosis in pregnancy. Am J Respir Crit Care Med 2012; 185:779–84. - PubMed
-
- Sobhy S, Babiker Z, Zamora J, Khan K, Kunst H. Maternal and perinatal mortality and morbidity associated with tuberculosis during pregnancy and the postpartum period: a systematic review and meta-analysis. BJOG 2017; 124:727–33. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

