The metabolic and physiologic impairments underlying long COVID associated exercise intolerance
- PMID: 39544193
- PMCID: PMC11560803
- DOI: 10.1002/pul2.70009
The metabolic and physiologic impairments underlying long COVID associated exercise intolerance
Abstract
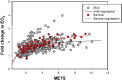
Data from invasive CPET (iCPET) revealed long COVID patients have impaired systemic oxygen extraction (EO2), suggesting impaired mitochondrial ATP production. However, it remains uncertain whether the initial severity of SARS-CoV-2 infection has implications on EO2 and exercise capacity (VO2) nor has there been assessment of anerobic ATP generation in long COVID patients. iCPET was performed on 47 long COVID patients (i.e., full cohort; n = 8 with severe SARS-CoV-2 infection). In a subset of patients (i.e., metabolomic cohort; n = 26) metabolomics on venous and arterial blood samples during iCPET was performed. In the full cohort, long COVID patients exhibited reduced peak EO2 with reduced peak VO2 (90 ± 17% predicted) relative to cardiac output (118 ± 23% predicted). Peak VO2 [88% predicted (IQR 81% - 108%) vs. 70% predicted (IQR 64% - 89%); p = 0.02] and EO2 [0.59(IQR 0.53-0.62) vs. 0.53(IQR 0.50-0.48); p = 0.01) were lower in severe versus mild infection. In the metabolomic cohort, 12 metabolites were significantly consumed, and 41 metabolites were significantly released (p-values < 0.05). Quantitative metabolomics demonstrated significant increases in inosine and succinate arteriovenous gradients during exercise. Peak VO2 was significantly correlated with peak venous succinate (r = 0.68; p = 0.0008) and peak venous lactate (r = 0.49; p = 0.0004). Peak EO2 and consequently peak VO2 impact long COVID patients in a severity dependent manner. Exercise intolerance associated with long COVID is defined by impaired aerobic and anaerobic energy production. Peak venous succinate may serve as a potential biomarker in long COVID.
Keywords: PASC; invasive CPET; long COVID; metabolomic.
© 2024 The Author(s). Pulmonary Circulation published by John Wiley & Sons Ltd on behalf of Pulmonary Vascular Research Institute.
Conflict of interest statement
BPL holds equity in EpiTET Therapeutics, Inc., which develops anti‐inflammatory treatments unrelated to thiswork. PMH has consulted for Cardiage LLC and Edwards Lifesciences, has received sponsored research funding from Edwards Lifesciences, and holds an equity interest in emka Medical. None of these relationships are related to the work presented.
Figures

References
-
- Organization WH. COVID‐19 epidemiological update . 2024;Accessed No. 168.). http://www.who.int/publications/m/item/covid-19-epidemiological-update-e...
-
- Joseph P, Singh I, Oliveira R, Capone CA, Mullen MP, Cook DB, Stovall MC, Squires J, Madsen K, Waxman AB, Systrom DM. Exercise pathophysiology in myalgic encephalomyelitis/chronic fatigue syndrome and postacute sequelae of SARS‐CoV‐2. Chest. 2023;164:717–726. 10.1016/j.chest.2023.03.049 - DOI - PMC - PubMed
-
- Durstenfeld MS, Sun K, Tahir P, Peluso MJ, Deeks SG, Aras MA, Grandis DJ, Long CS, Beatty A, Hsue PY. Use of cardiopulmonary exercise testing to evaluate long COVID‐19 symptoms in adults: A systematic review and meta‐analysis. JAMA Network Open. 2022;5:e2236057. 10.1001/jamanetworkopen.2022.36057 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

