Surveillance Imaging and GAAD/GALAD Scores for Detection of Hepatocellular Carcinoma in Patients with Chronic Hepatitis
- PMID: 39544249
- PMCID: PMC11557369
- DOI: 10.14218/JCTH.2024.00172
Surveillance Imaging and GAAD/GALAD Scores for Detection of Hepatocellular Carcinoma in Patients with Chronic Hepatitis
Abstract
Background and aims: Early detection of hepatocellular carcinoma (HCC) is crucial for improving survival in patients with chronic hepatitis. The GALAD algorithm combines gender (biological sex), age, α-fetoprotein (AFP), Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), and protein induced by vitamin K absence or antagonist-II (PIVKA-II) for HCC detection. Similarly, the GAAD algorithm incorporates gender (biological sex), age, AFP, and PIVKA-II. This study aimed to assess the clinical utility of AFP-L3 in the GALAD algorithm and its potential synergies with ultrasound. We compared the clinical performance of GALAD with GAAD; AFP; AFP-L3; and PIVKA-II, with or without ultrasound, in Taiwanese adults.
Methods: A total of 439 serum samples were analyzed using a Cobas® e 601 analyzer (healthy controls, n = 200; chronic liver disease controls, n = 177; HCC cases, n = 62). Performance was assessed through receiver operating characteristic curve analyses to calculate the area under the curve.
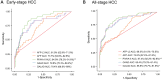
Results: The area under the curve for differentiating early-stage HCC from patients with chronic liver disease was optimal for PIVKA-II (84.9%), GAAD (79.8%), and GALAD (79.4%), with slightly improved performance for detecting all-stage HCC. Clinical performance was unaffected by disease stage or etiology. Sensitivity for early-stage HCC was highest for GAAD (57.6%) and GALAD (57.6%). Sensitivity for each strategy was further enhanced when combined with ultrasound, regardless of disease stage or etiology (P < 0.01).
Conclusions: These findings indicate that the role of AFP-L3 in the GALAD algorithm is minimal, supporting the use of GAAD for HCC detection. A combination of GAAD, GALAD, or PIVKA-II with ultrasound may improve diagnostic efficiency compared with recommended strategies.
Keywords: Algorithm; Biomarkers; Cirrhosis; Detection; Diagnosis; GAAD; GALAD; Hepatocellular carcinoma; Liver cancer; Surveillance; Ultrasound.
© 2024 Authors.
Conflict of interest statement
WLC: Member of advisory boards for Gilead, AbbVie, Vaccitech, PharmaEssentia; speaker for Gilead, AbbVie, BMS, Roche, and has been an Editorial Board Member of Journal of Clinical and Translational Hepatology since 2022. CFH: Speaker for AbbVie, BMS, Gilead, Merck, and Roche, JFH: Consultant for Roche, Gilead, Sysmex, and Aligos; speaker for AbbVie, Gilead, and Sysmex; and has been an Editorial Board Member of Journal of Clinical and Translational Hepatology since 2022. MLYu: Research grants from AbbVie, Gilead, Merck, and Roche Diagnostics; consultant for AbbVie, BMS, Gilead, Roche, and Roche Diagnostics; and speaker for AbbVie, BMS, Eisai, Gilead, Roche, and Roche Diagnostics; and has been an Associate Editor of Journal of Clinical and Translational Hepatology since 2023. AS is an employee of Roche Diagnostics International AG. KK is an employee of Roche Diagnostics GmbH. MLYeh has been an Editorial Board Member of Journal of Clinical and Translational Hepatology since 2022. The other authors have no conflict of interests related to this publication.
Figures



References
-
- World Health Organization. Data Visualization Tools for Exploring the Global Cancer Burden in 2020. Available from: http://gco.iarc.fr/today/home.
-
- Global Burden of Disease Liver Cancer Collaboration The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3(12):1683–1691. doi: 10.1001/jamaoncol.2017.3055. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
