Guidelines for Orbital Defect Assessment and Patient-Specific Implant Design: Introducing OA2 (Orbital Assessment Algorithm)
- PMID: 39544314
- PMCID: PMC11559584
- DOI: 10.1177/19433875241272436
Guidelines for Orbital Defect Assessment and Patient-Specific Implant Design: Introducing OA2 (Orbital Assessment Algorithm)
Abstract
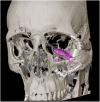
Study design: This study presents a review of the evolutionary development in reconstructive orbital surgery over the past 3 decades. Additionally, it proposes the Orbital Assessment Algorithm (OA2) to enhance decision-making for intraorbital reconstruction of post-traumatic orbital deformities.
Objective: The objective of this paper is to provide insights into modern post-traumatic orbital reconstruction from a surgeon's perspective, with a specific focus on adult patients. It aims to highlight the advancements in computer-aided design and manufacturing techniques, particularly in the field of reconstructive orbital surgery, and to introduce the OA2 as a tool for improved decision-making in this context.
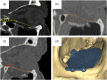
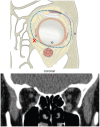
Methods: The study conducts a comprehensive review of the evolution of reconstructive orbital surgery, focusing on the integration of 3D technology into surgical practices. It also outlines the development and rationale behind the proposed OA2, emphasizing its potential to enhance the accuracy and efficacy of intraorbital reconstruction procedures for post-traumatic deformities.
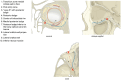
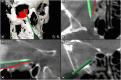
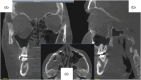
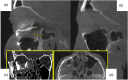
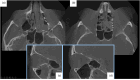
Results: The review demonstrates the significant progress made in reconstructive orbital surgery, particularly in leveraging 3D technology for virtual modeling, navigation, and the design and manufacturing of patient-specific implants. The introduction of the OA2 provides a structured approach to assessing and addressing post-traumatic orbital deformities, offering potential benefits in decision-making and surgical outcomes.
Conclusions: In conclusion, this paper underscores the pivotal role of computer-aided design and manufacturing in advancing reconstructive orbital surgery. It highlights the importance of integrating innovative design concepts into implant manufacturing processes and emphasizes the potential of the OA2 to guide surgeons in the management of post-traumatic orbital deformities, ultimately contributing to improved patient outcomes.
Keywords: functionalized implant design; orbital assessment algorithm; orbital reconstruction; patient-specific implants; post-traumatic orbital defect; virtual modelling.
© The Author(s) 2024.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors received speakers honoraria from DePuys Synthes®, West Chester, PA, USA, Brainlab AG, Munich, Germany and KLS Martin Group, Tuttlingen, Germany.
Figures






Similar articles
-
Three dimensional reconstruction of late post traumatic orbital wall defects by customized implants using CAD-CAM, 3D stereolithographic models: A case report.J Oral Biol Craniofac Res. 2017 Sep-Dec;7(3):212-218. doi: 10.1016/j.jobcr.2017.09.004. Epub 2017 Sep 14. J Oral Biol Craniofac Res. 2017. PMID: 29124002 Free PMC article.
-
Digital Technologies in the Surgical Treatment of Post-Traumatic Zygomatico-Orbital Deformities.Sovrem Tekhnologii Med. 2021;12(3):55-61. doi: 10.17691/stm2020.12.3.07. Epub 2020 Jun 28. Sovrem Tekhnologii Med. 2021. PMID: 34795980 Free PMC article.
-
Computer-assisted planning, stereolithographic modeling, and intraoperative navigation for complex orbital reconstruction: a descriptive study in a preliminary cohort.J Oral Maxillofac Surg. 2009 Dec;67(12):2559-70. doi: 10.1016/j.joms.2009.07.098. J Oral Maxillofac Surg. 2009. PMID: 19925972
-
Personalized Medicine Workflow in Post-Traumatic Orbital Reconstruction.J Pers Med. 2022 Aug 24;12(9):1366. doi: 10.3390/jpm12091366. J Pers Med. 2022. PMID: 36143151 Free PMC article. Review.
-
Virtual Planning and 3D Printing in the Management of Acute Orbital Fractures and Post-Traumatic Deformities.Semin Plast Surg. 2022 Dec 7;36(3):149-157. doi: 10.1055/s-0042-1754387. eCollection 2022 Aug. Semin Plast Surg. 2022. PMID: 36506274 Free PMC article. Review.
Cited by
-
Patient-Specific Solutions for Cranial, Midface, and Mandible Reconstruction Following Ablative Surgery: Expert Opinion and a Consensus on the Guidelines and Workflow.Craniomaxillofac Trauma Reconstr. 2025 Feb 13;18(1):15. doi: 10.3390/cmtr18010015. eCollection 2025 Mar. Craniomaxillofac Trauma Reconstr. 2025. PMID: 40271473 Free PMC article.
References
-
- Manson PN. Three-dimensional CT diagnosis of maxillofacial trauma. N Engl J Med. 1994;330(1):69. - PubMed
-
- Zimmerer RM, Ellis E, 3rd, Aniceto GS, et al. A prospective multicenter study to compare the precision of posttraumatic internal orbital reconstruction with standard preformed and individualized orbital implants. J Cranio-Maxillo-Fac Surg. 2016;44(9):1485-1497. doi:10.1016/j.jcms.2016.07.014 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
