Liver Transplantation for Intrahepatic Cholangiocarcinoma After Chemotherapy and Radioembolization: An Intention-To-Treat Study
- PMID: 39544321
- PMCID: PMC11560448
- DOI: 10.3389/ti.2024.13641
Liver Transplantation for Intrahepatic Cholangiocarcinoma After Chemotherapy and Radioembolization: An Intention-To-Treat Study
Abstract
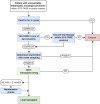
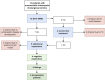
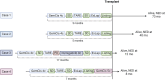
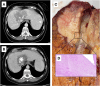
Liver transplantation (LT) is a potentially curative experimental treatment for unresectable intrahepatic cholangiocarcinoma (iCC). Pre-transplant downstaging may help defining tumor aggressiveness and drive patient selection. We report the preliminary results of LT for liver-limited unresectable iCC after sequential downstaging with systemic chemotherapy and radioembolization (SYS-TARE). In case of sustained disease stability after SYS-TARE, patients underwent surgical nodal sampling and, if negative, were listed for LT. In this study, 13 patients with unresectable iCC underwent downstaging with SYS-TARE. The median age was 70 years and 77% were female. All had single bulky lesions at diagnosis. After SYS-TARE, 9 (69%) dropped out: 3 due to progressive disease after TARE with no response to second-line, 4 due to extrahepatic disease development and 2 due to positive nodal disease at pre-listing abdominal exploration. The median OS after dropout was 11.5 months. Four (31%) were successfully listed and transplanted. At pathology, viable tumor ranged from 30% to less than 5%. All four patients are alive and disease-free at 73, 40, 12, and 8 months from LT. LT for unresectable iCC after downstaging with SYS-TARE appears to select suitable patients for LT, achieving optimal oncological outcomes in case of response to therapy and no lymphnodal spread.
Keywords: Yttrium-90; biliary tract cancers; downstaging; gemcitabine-cisplatin; tare.
Copyright © 2024 Maspero, Sposito, Bongini, Cascella, Flores, Maccauro, Chiesa, Niger, Pietrantonio, Leoncini, Bellia, Bhoori and Mazzaferro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Oh D-Y, He AR, Bouattour M, Okusaka T, Qin S, Chen L-T, et al. Durvalumab or Placebo Plus Gemcitabine and Cisplatin in Participants with Advanced Biliary Tract Cancer (TOPAZ-1): Updated Overall Survival from a Randomised Phase 3 Study. Lancet Gastroenterol Hepatol (2024) 9(8):694–704. 10.1016/S2468-1253(24)00095-5 - DOI - PubMed

