Assessment of Bone Loss Around Platform-Switching Subcrestal Implants: A Quasi-Experimental Study
- PMID: 39544579
- PMCID: PMC11560384
- DOI: 10.7759/cureus.71460
Assessment of Bone Loss Around Platform-Switching Subcrestal Implants: A Quasi-Experimental Study
Abstract
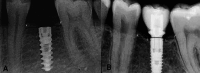
Introduction: Replacement of lost teeth with implants is a well-known and accepted worldwide treatment. A healthy amount of bone surrounding the implant plays a vital role in osseointegration and is required for implant success. This study aimed to evaluate the efficacy of single-crown rehabilitation of subcrestal implants in terms of bone loss (BL) and overall success.
Materials and methods: Twenty healthy patients requiring implant placement to replace single hopeless teeth or extracted teeth were recruited for this prospective study. In these 20 patients, the implants were placed at the subcrestal level. Bone levels around the implant placement (T0) and one year after loading (T1) were estimated in this study. A paired t-test was used for intra-group comparisons, multivariate linear regression analysis was conducted to analyze the effect of independent variables on BL, and a correlation test was used to correlate various variables. Statistical significance was maintained at a p value of 0.05.
Results: The outcomes demonstrated statistically significant BL at the mesial and distal sides of the subcrestal implants at one-year follow-up (p<0.05). Age and sex were not significantly correlated with BL in any region (p>0.05). Brushing frequency, probing depth (PD), and bleeding index (BI) showed statistically significant effects on BL (p<0.05). A weak correlation was observed between age and other variables, with age and mesial BL showing a low correlation of 0.016.
Conclusion: Within the parameters of this prospective study, it could be proposed that subcrestal implants caused significant BL. The PD, BI, and brushing frequency were significant predictors of BL.
Keywords: bone loss; brushing; dental implants; follow up; periodontal index; stability.
Copyright © 2024, Yadav et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Institutional Ethical Committee Maharaja Ganga Singh Dental College issued approval MGSDC/SY/22/018. Institutional ethics committee approval (MGSDC/SY/22/018) was obtained before starting the study, and the study was conducted in accordance with the principles of the Declaration of Helsinki. Written consent was obtained from all participants prior to the study. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Research on implants and osseointegration. Guglielmotti MB, Olmedo DG, Cabrini RL. Periodontol 2000. 2019;79:178–189. - PubMed
-
- Equicrestal and subcrestal dental implants: a histologic and histomorphometric evaluation of nine retrieved human implants. Degidi M, Perrotti V, Shibli JA, Novaes AB, Piattelli A, Iezzi G. J Periodontol. 2011;82:708–715. - PubMed
LinkOut - more resources
Full Text Sources
