Preclinical evaluation of the CD38-targeting engineered toxin body MT-0169 against multiple myeloma
- PMID: 39544624
- PMCID: PMC11561653
- DOI: 10.1002/hem3.70039
Preclinical evaluation of the CD38-targeting engineered toxin body MT-0169 against multiple myeloma
Abstract
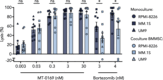
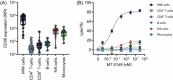
Despite significant progress in the treatment of multiple myeloma (MM), relapsed/refractory patients urgently require more effective therapies. We here describe the discovery, mechanism of action, and preclinical anti-MM activity of engineered toxin body MT-0169, a next-generation immunotoxin comprising a CD38-specific antibody fragment linked to a de-immunized Shiga-like toxin A subunit (SLTA) payload. We show that specific binding of MT-0169 to CD38 on MM cell lines triggers rapid internalization of SLTA, causing cell death via irreversible ribosome inhibition, protein synthesis blockade, and caspase 3/7 activation. In co-culture experiments, bone marrow mesenchymal stromal cells did not induce drug resistance against MT-0169. In the preclinical setting, MT-0169 effectively lysed primary MM cells from newly diagnosed and heavily pretreated MM patients, including those refractory to daratumumab, with minimal toxicity against nonmalignant hematopoietic cells. MM cell lysis showed a significant correlation with their CD38 expression levels but not with cytogenetic risk, tumor load, or number of prior lines of therapy. Finally, MT-0169 showed efficient in vivo anti-MM activity in various mouse xenograft models, including one in which MM cells are grown in a humanized bone marrow-like niche. These findings support clinical investigation of MT-0169 in relapsed/refractory MM patients, including those refractory to CD38-targeting immunotherapies.
© 2024 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
John Newcomb is a former employee of Takeda Development Center Americas, Inc., and a current employee of Dynamic Cell Therapies, Inc. Cambridge, MA, USA; Eric Poma is a current employee of Molecular Templates, Inc., and currently runs clinical oncology trials with MT‐0169. Chris Moore is a current employee of Molecular Templates, Inc., and currently runs clinical oncology trials with MT‐0169. Garrett L. Robinson is a current employee of Molecular Templates, Inc. Anya Lublinsky is a current employee and stockholder of Takeda Development Center Americas, Inc. Yuhong Zhang is a current employee and stockholder of Takeda Development Center Americas, Inc. Sakeena Syed is a former employee and stockholder of Takeda Development Center Americas, Inc. Michael Milhollen is a current employee and stockholder of Takeda Development Center Americas, Inc. Ajeeta B. Dash is a current employee and stockholder of Takeda Development Center Americas, Inc. Niels W. C. J. van de Donk has received research support from Janssen Pharmaceuticals, AMGEN, Celgene, Novartis, Cellectis, and BMS. He serves on advisory boards for Janssen Pharmaceuticals, AMGEN, Celgene, BMS, Takeda, Roche, Novartis, Bayer, Pfizer, Abbvie, Adaptive, and Servier, all paid to employer. Richard W. J. Groen has received research support from Takeda. Sonja Zweegman has received research support from Celgene, Takeda, and Janssen Pharmaceuticals. She serves on advisory boards for Celgene, Takeda, Janssen Pharmaceuticals, Sanofi, Amgen, and Oncopeptides (no personal funding). Tuna Mutis has received research support from Janssen Pharmaceuticals, Takeda, Genmab, Novartis, and ONK Therapeutics. For the remaining authors, no relevant conflicts of interest were declared.
Figures




References
-
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:m3176. - PubMed
-
- Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373(13):1207‐1219. - PubMed
-
- Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem‐cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open‐label, phase 3 study. Lancet. 2019;394(10192):29‐38. - PubMed
-
- Munshi NC, Anderson LD Jr., Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705‐716. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
