Reduced guanidinoacetate in plasma of patients with autosomal dominant Fanconi syndrome due to heterozygous P341L GATM variant and study of organoids towards treatment
- PMID: 39544690
- PMCID: PMC11558468
- DOI: 10.1002/jmd2.12442
Reduced guanidinoacetate in plasma of patients with autosomal dominant Fanconi syndrome due to heterozygous P341L GATM variant and study of organoids towards treatment
Abstract
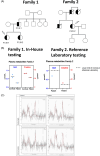
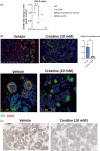
Autosomal dominant Fanconi syndrome due to a GATM variant (GATM-FS), causes accumulation of misfolded arginine-glycine amidinotransferase (AGAT) in proximal renal tubules leading to cellular injury. GATM-FS presents during childhood and progresses to end-stage kidney disease (ESKD) in adults. We study creatine metabolism in two individuals of unrelated families with a known GATM variant and the effect of creatine supplementation in kidney organoids. Plasma and urine metabolites were measured by mass spectrometry. Brain creatine was assessed by magnetic resonance spectroscopy (MRS). Guanidinoacetate (GAA) synthesis by the AGAT mutant was measured in patient-derived immortalized lymphocytes using stable isotopes of arginine and glycine. The effect of creatine on GATM expression was assessed in human kidney cells and organoids. Several family members from two unrelated families were diagnosed with Fanconi syndrome and had the c.1022C>T (p. P341L) variant in GATM. Two affected individuals in both families had moderately reduced plasma GAA levels. In comparison to wild-type cells, GAA synthesis by patient-derived GATM P341L+/- lymphoblastoid cell lines (LCL) was reduced, but not absent as in GATM cells from a patient with creatine deficiency syndrome. In vitro studies on human kidney organoids revealed reduced AGAT expression after treatment with creatine. Finally, we showed in one patient that creatine supplementation (5 g daily) substantially increased plasma creatine levels. We report low plasma and urine GAA in patients with autosomal dominant GATM-FS and show that creatine downregulates AGAT in human kidney cells.
Keywords: Fanconi syndrome; GATM; arginine‐glycine amidinotransferase (AGAT); chronic kidney disease; creatine; guanidinoacetate; kidney organoids.
© 2024 The Author(s). JIMD Reports published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Ignacio Portales‐Castillo, Rhea Singal, Anastasia Ambrose, Jong Hee Song, Minsoo Son, Young Ah Goo, Wen Zhou, Avram Z. Traum, Ariella Coller‐Reilly, Benjamin D. Humphreys, Roberto Civitelli, Harald Jüppner, Andrew L. Lundquist, Peter Seres, Andrew S. Allegretti and Saadet Mercimek‐Andrews declare that they have no conflict of interest.
Figures

References
-
- Ragate DC, Memon SS, Karlekar M, et al. Inherited Fanconi renotubular syndromes: unveiling the intricacies of hypophosphatemic rickets/osteomalacia. J Bone Miner Metab. 2024;42(2):155‐165. - PubMed
-
- Li C‐Y, Sun Y, Guo W‐C, et al. Complex phenotype in Fanconi renotubular syndrome type 1: Hypophosphatemic rickets as the predominant presentation. Clin Chim Acta. 2024;561:119812. - PubMed
-
- Kudo H, Suzuki R, Kondo A, et al. Association of familial Fanconi syndrome with a novel GATM variant. Tohoku J Exp Med. 2023;260(4):337‐340. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

