Comparative pharmacokinetics of porcine and human anti-influenza hemagglutinin monoclonal antibodies in outbred pigs and minipigs
- PMID: 39544926
- PMCID: PMC11560753
- DOI: 10.3389/fimmu.2024.1471412
Comparative pharmacokinetics of porcine and human anti-influenza hemagglutinin monoclonal antibodies in outbred pigs and minipigs
Abstract
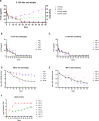
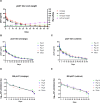
Assessing the pharmacokinetics of monoclonal antibodies (mAbs) in relevant animal models is essential for designing improved formulations and developing mAb delivery platforms. We have established the pig, a large natural host animal for influenza with many similarities to humans, as a robust model for testing the therapeutic efficacy of anti-influenza mAbs and evaluating mAb delivery platforms. Here, we compared the pharmacokinetic characteristics of two anti-influenza hemagglutinin mAbs, human 2-12C and porcine pb27, in Göttingen minipigs and Landrace × Large White outbred pigs. Minipigs offer the advantage of a more stable weight, whereas outbred pigs are more readily available but exhibit rapid growth. Outbred pigs and minipigs showed similar pharmacokinetics and a similar porcine pb27 half-life (half-life of 15.7 days for outbred pigs and 16.6 days for minipigs). In contrast, the half-life of human 2-12C was more rapid in two of the minipigs but not in the outbred pigs, correlating with the development of antidrug antibodies in the two minipigs. Our results demonstrate that both outbred pigs and minipigs are appropriate models for pharmacokinetic studies and the evaluation of mAb delivery platforms, potentially bridging the gap between small animals and human trials.
Keywords: 2-12C; anti-influenza monoclonal antibodies; minipigs; outbred pigs; pb27; pharmacokinetic.
Copyright © 2024 Paudyal, Moorhouse, Sharma, Dodds, Nguyen, Milad and Tchilian.
Conflict of interest statement
MD was employed by the company Certara. VN and MM were employed by the company Milad Pharmaceutical Consulting LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Establishment of a Pig Influenza Challenge Model for Evaluation of Monoclonal Antibody Delivery Platforms.J Immunol. 2020 Aug 1;205(3):648-660. doi: 10.4049/jimmunol.2000429. Epub 2020 Jun 26. J Immunol. 2020. PMID: 32591390 Free PMC article.
-
A direct contact pig influenza challenge model for assessing protective efficacy of monoclonal antibodies.Front Immunol. 2023 Oct 27;14:1229051. doi: 10.3389/fimmu.2023.1229051. eCollection 2023. Front Immunol. 2023. PMID: 37965320 Free PMC article.
-
Low Dose Pig Anti-Influenza Virus Monoclonal Antibodies Reduce Lung Pathology but Do Not Prevent Virus Shedding.Front Immunol. 2021 Dec 16;12:790918. doi: 10.3389/fimmu.2021.790918. eCollection 2021. Front Immunol. 2021. PMID: 34975888 Free PMC article.
-
Influenza Hemagglutinin Structures and Antibody Recognition.Cold Spring Harb Perspect Med. 2020 Aug 3;10(8):a038778. doi: 10.1101/cshperspect.a038778. Cold Spring Harb Perspect Med. 2020. PMID: 31871236 Free PMC article. Review.
-
Development of next generation hemagglutinin-based broadly protective influenza virus vaccines.Curr Opin Immunol. 2018 Aug;53:51-57. doi: 10.1016/j.coi.2018.04.001. Epub 2018 Apr 20. Curr Opin Immunol. 2018. PMID: 29680576 Review.
References
-
- Merchant HA, McConnell EL, Liu F, Ramaswamy C, Kulkarni RP, Basit AW, et al. . Assessment of gastrointestinal pH, fluid and lymphoid tissue in the Guinea pig, rabbit and pig, and implications for their use in drug development. Eur J Pharm Sci. (2011) 42:3–10. doi: 10.1016/j.ejps.2010.09.019 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

