The microglial innate immune receptor TREM2 participates in fear memory formation through excessive prelimbic cortical synaptic pruning
- PMID: 39544929
- PMCID: PMC11560470
- DOI: 10.3389/fimmu.2024.1412699
The microglial innate immune receptor TREM2 participates in fear memory formation through excessive prelimbic cortical synaptic pruning
Abstract
Introduction: Fear memory formation has been implicated in fear- and stress-related psychiatric disorders, including post-traumatic stress disorder (PTSD) and phobias. Synapse deficiency and microglial activation are common among patients with PTSD, and induced in animal models of fear conditioning. Increasing studies now focus on explaining the specific mechanisms between microglia and synapse deficiency. Though newly-identified microglia regulator triggering receptor expressed on myeloid cells 2 (TREM2) plays a role in microglial phagocytic activity, its role in fear-formation remains unknown.
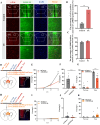
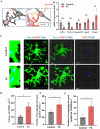
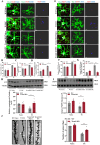
Methods: We successfully constructed a fear- formation model by foot-shock. Four days after foot-shock, microglial capacity of synaptic pruning was investigated via western blotting, immunofluorescence and Golgi-Cox staining. Prelimbic chemical deletion or microglia inhibition was performed to detect the role of microglia in synaptic loss and neuron activity. Finally, Trem2 knockout mice or wild-type mice with Trem2 siRNA injection were exposed to foot-shock to identify the involvement of TREM2 in fear memory formation.
Results: The results herein indicate that the foot-shock protocol in male mice resulted in a fear formation model. Mechanistically, fear conditioning enhanced the microglial capacity for engulfing synapse materials, and led to glutamatergic neuron activation in the prelimbic cortex. Prelimbic chemical deletion or microglia inhibition improved fear memory formation. Further investigation demonstrated that TREM2 regulates microglial phagocytosis, enhancing synaptic pruning. Trem2 knockout mice showed remarkable reductions in prelimbic synaptic pruning and reduced neuron activation, with decreased fear memory formation.
Discussion: Our cumulative results suggest that prelimbic TREM2-mediated excessive microglial synaptic pruning is involved in the fear memory formation process, leading to development of abnormal stress-related behavior.
Keywords: TREM2; fear memory formation; microglia; prelimbic; synaptic pruning.
Copyright © 2024 Zhang, Cheng, Chu, Zhou, Hua and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
TREM2 regulates microglial phagocytosis of synapses in innate immune tolerance.Int Immunopharmacol. 2024 Jan 25;127:111445. doi: 10.1016/j.intimp.2023.111445. Epub 2023 Dec 25. Int Immunopharmacol. 2024. PMID: 38147777
-
The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity.Immunity. 2018 May 15;48(5):979-991.e8. doi: 10.1016/j.immuni.2018.04.016. Epub 2018 May 8. Immunity. 2018. PMID: 29752066
-
Triggering Receptor Expressed on Myeloid Cells 2 Alleviated Sevoflurane-Induced Developmental Neurotoxicity via Microglial Pruning of Dendritic Spines in the CA1 Region of the Hippocampus.Neurosci Bull. 2024 Sep;40(9):1215-1229. doi: 10.1007/s12264-024-01260-9. Epub 2024 Jul 29. Neurosci Bull. 2024. PMID: 39078595 Free PMC article.
-
TREM2 and Microglia Contribute to the Synaptic Plasticity: from Physiology to Pathology.Mol Neurobiol. 2023 Feb;60(2):512-523. doi: 10.1007/s12035-022-03100-1. Epub 2022 Nov 1. Mol Neurobiol. 2023. PMID: 36318443 Review.
-
Microglia and Aging: The Role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes.Int J Mol Sci. 2018 Jan 22;19(1):318. doi: 10.3390/ijms19010318. Int J Mol Sci. 2018. PMID: 29361745 Free PMC article. Review.
Cited by
-
Research Progress on Inflammation and Immune Dysregulation in PTSD.Brain Behav. 2025 Jun;15(6):e70633. doi: 10.1002/brb3.70633. Brain Behav. 2025. PMID: 40525295 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

