Dexamethasone and IFN-γ primed mesenchymal stem cells conditioned media immunomodulates aberrant NETosis in SLE via PGE2 and IDO
- PMID: 39544931
- PMCID: PMC11560778
- DOI: 10.3389/fimmu.2024.1461841
Dexamethasone and IFN-γ primed mesenchymal stem cells conditioned media immunomodulates aberrant NETosis in SLE via PGE2 and IDO
Abstract
Background: Systemic Lupus Erythematosus (SLE) is characterized by dysregulated immune responses, with neutrophil extracellular traps (NETs) playing a significant role. NETs are recognized by autoantibodies in SLE patients, exacerbating pathology. Both excessive NET formation and impaired degradation contribute to SLE pathophysiology.
Objective: To investigate the immunomodulatory effects of Dexamethasone-primed Wharton's jelly (WJ) derived MSCs CM (DW) and IFN-γ-primed WJ-MSCs-CM (IW) on NETosis and associated protein markers in SLE patients' LPS or ribonucleoprotein immune complexes (RNP ICs) induced neutrophils and in pristane induced lupus (PIL) model. And to elucidate the mechanism involved therein.
Methods: We investigated the immunomodulatory effects of DW and IW on NETosis in SLE. Utilizing ex vivo and in vivo models, we assessed the impact of preconditioned media on NET formation and associated protein markers neutrophil elastase (NE), citrullinated histone (citH3), myeloperoxidase (MPO), cytoplasmic and mitochondrial ROS production. We also examined the involvement of key immunomodulatory factors present in DW and IW, including prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase (IDO), and transforming growth factor-beta (TGF-β).
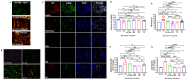
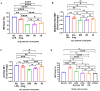
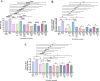
Results: Preconditioned media effectively suppressed NETosis and reduced ROS generation in SLE neutrophils, indicating their immunomodulatory potential. Inhibition studies implicated IDO and PGE2 in mediating this effect. Combined treatment with DW or IW together with hydroxychloroquine (HCQ) demonstrated superior efficacy over HCQ alone, a standard SLE medication. In PIL mouse model, DW and IW treatments reduced NETosis, ROS generation, as evidenced by decreased NET-associated protein expression in vital organs.
Conclusion: Our study highlights the multifaceted impact of IW and DW on NETosis, ROS dynamics, and lupus severity in SLE. These findings underscore the potential of preconditioned media for the development of targeted, personalized approaches for SLE treatment.
Keywords: NETosis; hydroxychloroquine; lupus model; neutrophil extracellular trap formation; reactive oxygen species; ribonucleoprotein immune complexes; systemic lupus erythematosus.
Copyright © 2024 Priya, Thacker, Chaubey, Rai, Singh, Rawat, Giri, Mohanty and Rai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Fatemi A, Alipour R, Khanahmad H, Alsahebfosul F, Andalib A, Pourazar A. The impact of neutrophil extracellular trap from patients with systemic lupus erythematosus on the viability, CD11b expression and oxidative burst of healthy neutrophils. BMC Immunol. (2021) 22:12. doi: 10.1186/s12865-021-00402-2 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

