Comparison of the efficacy and safety of neoadjuvant PD-1 inhibitors plus chemotherapy vs targeted therapy plus chemotherapy in locally advanced hypopharyngeal squamous cell carcinoma
- PMID: 39544946
- PMCID: PMC11560446
- DOI: 10.3389/fimmu.2024.1466310
Comparison of the efficacy and safety of neoadjuvant PD-1 inhibitors plus chemotherapy vs targeted therapy plus chemotherapy in locally advanced hypopharyngeal squamous cell carcinoma
Abstract
Objective: To investigate the clinical efficacy, preservation of laryngeal function, and safety differences between PD-1 inhibitors combined with chemotherapy, and targeted therapy combined with chemotherapy in LA HPSCC patients.
Methods: This was a retrospective analysis of patients with LA HPSCC treated at Beijing Tongren Hospital, Capital Medical University from October, 2020 to March, 2024. A total of 110 eligible patients were included, 56 in the PD-1 inhibitors combined with chemotherapy group (Group A), and 54 in the targeted therapy combined with chemotherapy group (Group B). Relevant clinical data were collected, and the clinical efficacy, preservation of laryngeal function, complete response (CR) rate, pathological complete response (pCR) rate, major pathological response (MPR), and treatment-related adverse events (TRAEs) of the two groups were analyzed and compared.
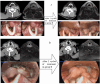
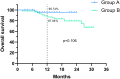
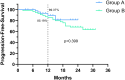
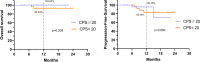
Results: In both groups A and B, the objective response rate (ORR) and disease control rate (DCR) were similar with no significant differences, but the pCR rate in Group A was much higher than that in Group B, at 37.5% and 7.4%, respectively (p<0.001). The rate of primary tumor downstaging in group A was much higher than that in group B (76.8% vs. 38.9%) as well (p<0.0001). In addition, the 1y-OS rate in group A was 95.7%, compared to 87.0% in group B (p=0.106, HR=0.34; 95% CI: 0.114-1.013), and the 1y-PFS rate was 89.4% in group A compared to 85.2% in group B (p=0.399, HR=0.675; 95% CI: 0.275-1.659). Furthermore, the larynx function preservation rate was significantly higher in group A at 85.7%, compared to that of group B at only 66.7% (p=0.019). There were no deaths due to TRAEs in either group, and there was no significant difference in the incidence of grade 3-4 TRAEs between the two groups either (p=0.77). The main TRAEs in Group A were metabolism and nutrition disorders (52/56, 92.9%) and, in Group B were blood and lymphatic system disorders (40/54, 74.1%).
Conclusions: PD-1 inhibitors combined with chemotherapy showed better short-term efficacy compared to targeted therapy. Additionally, a trend toward improved long-term survival was observed with PD-1 inhibitors but not with targeted therapy. Results for both groups indicate that neoadjuvant therapy is both safe and manageable.
Keywords: PD-1 inhibitors; comparison of efficacy; drug response; hypopharyngeal squamous cell carcinoma; immunotherapy.
Copyright © 2024 Gao, Feng, Zhao, Huang, Chen, Yin, Guo, Zhong, Chen, Fang, Zhang and Huang.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Neoadjuvant immunochemotherapy in locally advanced laryngeal cancer and hypopharyngeal cancer: higher objective response rate and organ-preservation rate.Invest New Drugs. 2024 Dec;42(6):694-702. doi: 10.1007/s10637-024-01456-w. Epub 2024 Nov 28. Invest New Drugs. 2024. PMID: 39607584
-
PD-1 inhibitor combined with paclitaxel and cisplatin in the treatment of recurrent and metastatic hypopharyngeal/laryngeal squamous cell carcinoma: efficacy and survival outcomes.Front Immunol. 2024 May 17;15:1353435. doi: 10.3389/fimmu.2024.1353435. eCollection 2024. Front Immunol. 2024. PMID: 38827739 Free PMC article.
-
[Efficacy of PD-1 inhibitors combined with nab-paclitaxel and cisplatin in the neoadjuvant treatment of locally advanced hypopharyngeal squamous cell carcinoma].Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024 Jul 7;59(7):750-757. doi: 10.3760/cma.j.cn115330-20231016-00152. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024. PMID: 39107124 Chinese.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
Cited by
-
Global, regional and national burden and trends of larynx cancer among adults aged 55 and older from 1990 to 2021: results from the global burden of disease study 2021.BMC Public Health. 2025 Mar 6;25(1):906. doi: 10.1186/s12889-025-21993-0. BMC Public Health. 2025. PMID: 40050798 Free PMC article.
-
Efficacy and toxicity of PD-1 inhibitor combined with induction chemotherapy for locally advanced laryngeal and hypopharyngeal cancers.Am J Cancer Res. 2025 May 15;15(5):2193-2207. doi: 10.62347/HVRH6856. eCollection 2025. Am J Cancer Res. 2025. PMID: 40520879 Free PMC article.
-
Neoadjuvant treatment in locally advanced thyroid cancer: a single institution experience.Front Oncol. 2025 Jun 17;15:1590086. doi: 10.3389/fonc.2025.1590086. eCollection 2025. Front Oncol. 2025. PMID: 40599855 Free PMC article.
References
-
- Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. (1996) 88:890–9. doi: 10.1093/jnci/88.13.890 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

