Growth of soil ammonia-oxidizing archaea on air-exposed solid surface
- PMID: 39544964
- PMCID: PMC11561398
- DOI: 10.1093/ismeco/ycae129
Growth of soil ammonia-oxidizing archaea on air-exposed solid surface
Abstract
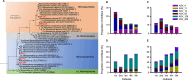
Soil microorganisms often thrive as microcolonies or biofilms within pores of soil aggregates exposed to the soil atmosphere. However, previous studies on the physiology of soil ammonia-oxidizing microorganisms (AOMs), which play a critical role in the nitrogen cycle, were primarily conducted using freely suspended AOM cells (planktonic cells) in liquid media. In this study, we examined the growth of two representative soil ammonia-oxidizing archaea (AOA), Nitrososphaera viennensis EN76 and "Nitrosotenuis chungbukensis" MY2, and a soil ammonia-oxidizing bacterium, Nitrosomonas europaea ATCC 19718 on polycarbonate membrane filters floated on liquid media to observe their adaptation to air-exposed solid surfaces. Interestingly, ammonia oxidation activities of N. viennensis EN76 and "N. chungbukensis" MY2 were significantly repressed on floating filters compared to the freely suspended cells in liquid media. Conversely, the ammonia oxidation activity of N. europaea ATCC 19718 was comparable on floating filters and liquid media. N. viennensis EN76 and N. europaea ATCC 19718 developed microcolonies on floating filters. Transcriptome analysis of N. viennensis EN76 floating filter-grown cells revealed upregulation of unique sets of genes for cell wall and extracellular polymeric substance biosynthesis, ammonia oxidation (including ammonia monooxygenase subunit C (amoC3) and multicopper oxidases), and defense against H2O2-induced oxidative stress. These genes may play a pivotal role in adapting AOA to air-exposed solid surfaces. Furthermore, the floating filter technique resulted in the enrichment of distinct soil AOA communities dominated by the "Ca. Nitrosocosmicus" clade. Overall, this study sheds light on distinct adaptive mechanisms governing AOA growth on air-exposed solid surfaces.
Keywords: H2O2-induced oxidative stress; air-exposed solid surfaces; floating filter cultivation; soil ammonia-oxidizing archaea; soil nitrification; transcriptome.
© The Author(s) 2024. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
The authors declare no competing interests.
Figures


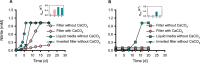


Similar articles
-
Cultivation of ammonia-oxidising archaea on solid medium.FEMS Microbiol Lett. 2022 May 5;369(1):fnac029. doi: 10.1093/femsle/fnac029. FEMS Microbiol Lett. 2022. PMID: 35323924 Free PMC article.
-
Responses of the terrestrial ammonia-oxidizing archaeon Ca. Nitrososphaera viennensis and the ammonia-oxidizing bacterium Nitrosospira multiformis to nitrification inhibitors.FEMS Microbiol Lett. 2013 Jul;344(2):121-9. doi: 10.1111/1574-6968.12164. Epub 2013 May 20. FEMS Microbiol Lett. 2013. PMID: 23617238
-
Genomic profiling of soil nitrifying microorganisms enriched on floating membrane filter.J Microbiol. 2025 Apr;63(4):e2502002. doi: 10.71150/jm.2502002. Epub 2025 Apr 29. J Microbiol. 2025. PMID: 40313154
-
Selective Enrichment of Nitrososphaera viennensis-Like Ammonia-Oxidizing Archaea over Ammonia-Oxidizing Bacteria from Drinking Water Biofilms.Microbiol Spectr. 2022 Dec 21;10(6):e0184522. doi: 10.1128/spectrum.01845-22. Epub 2022 Nov 29. Microbiol Spectr. 2022. PMID: 36445127 Free PMC article.
-
Copper limiting threshold in the terrestrial ammonia oxidizing archaeon Nitrososphaera viennensis.Res Microbiol. 2020 Apr-Jun;171(3-4):134-142. doi: 10.1016/j.resmic.2020.01.003. Epub 2020 Jan 25. Res Microbiol. 2020. PMID: 31991171
References
LinkOut - more resources
Full Text Sources

