First case report of a vertebral osteomyelitis caused by carbapenem-resistant Enterobacter cloacae treated with imipenem/cilastatin/relebactam prolonged infusion then meropenem/vaborbactam in continuous infusion
- PMID: 39545060
- PMCID: PMC11561750
- DOI: 10.3389/fphar.2024.1347306
First case report of a vertebral osteomyelitis caused by carbapenem-resistant Enterobacter cloacae treated with imipenem/cilastatin/relebactam prolonged infusion then meropenem/vaborbactam in continuous infusion
Abstract
Introduction: Bone and joint infections (BJIs) caused by multidrug-resistant bacteria are becoming more frequent. However, data on the use of novel β-lactam/β-lactamase inhibitors, such as imipenem/cilastatin/relebactam (I-R) and meropenem/vaborbactam (MVB), to treat BJIs is lacking. Furthermore, prolonged infusions of these β-lactams should theoretically optimize pharmacokinetic/pharmacodynamics target in these indications, but there are currently no reports on this type of infusions, especially in the setting of BJI.
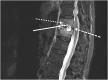
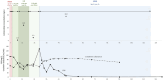
Case presentation: We report a case of a vertebral osteomyelitis caused by carbapenem-resistant Enterobacter cloacae successfully treated with extended-infusion of I-R (1.25 g q6h over 2 h), then with continuous infusion of MVB (2 g q4h as over 4 h). Therapeutic drug monitoring confirmed that extended-infusion of I-R and continuous infusion of MVB achieved serum concentrations up to 12 mg/L of imipenem and 19 mg/L of meropenem, respectively.
Conclusion: The favourable outcome of this patient treated for a vertebral osteomyelitis caused by carbapenem-resistant E. cloacae suggest that extended- and continuous infusions of I-R and MVB, are promising regimens for treatment of BJIs caused by carbapenem-resistant Enterobacterales.
Keywords: bone and joint infection; continuous infusion; extended infusion; imipenem/cilastatin/relebactam; meropenem/vaborbactam; therapeutic drug monitoring; vertebral osteomyelitis.
Copyright © 2024 Laffont-Lozes, Naciri, Pantel, Martin, Pruvot-Occean, Haignere, Loubet, Sotto and Larcher.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bouglé A., Dujardin O., Lepère V., Ait Hamou N., Vidal C., Lebreton G., et al. (2019). PHARMECMO: therapeutic drug monitoring and adequacy of current dosing regimens of antibiotics in patients on Extracorporeal Life Support. Anaesth. Crit. Care and Pain Med. 38, 493–497. 10.1016/j.accpm.2019.02.015 - DOI - PubMed
-
- Brown M. L., Motsch J., Kaye K. S., File T. M., Boucher H. W., Vendetti N., et al. (2020). Evaluation of renal safety between imipenem/relebactam and colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections in the randomized, phase 3 RESTORE-IMI 1 study. Open Forum Infect. Dis. 7, ofaa054. 10.1093/ofid/ofaa054 - DOI - PMC - PubMed
-
- Davido B., Crémieux A.-C., Vaugier I., Gatin L., Noussair L., Massias L., et al. (2023). Efficacy of ceftazidime-avibactam in various combinations for the treatment of experimental osteomyelitis due to Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae . Int. J. Antimicrob. Agents 61, 106702. 10.1016/j.ijantimicag.2022.106702 - DOI - PubMed
-
- Katip W., Rayanakorn A., Oberdorfer P., Taruangsri P., Nampuan T., Okonogi S. (2024). Comparative effectiveness and mortality of colistin monotherapy versus colistin-fosfomycin combination therapy for the treatment of carbapenem-resistant Enterobacteriaceae (CRE) infections: a propensity score analysis. J. Infect. Public Health 17, 727–734. 10.1016/j.jiph.2024.03.010 - DOI - PubMed
LinkOut - more resources
Full Text Sources

