Isatin improves oligoasthenospermia caused by busulfan by regulating GSH/GPX4 axis to inhibit ferroptosis
- PMID: 39545065
- PMCID: PMC11561459
- DOI: 10.3389/fphar.2024.1489956
Isatin improves oligoasthenospermia caused by busulfan by regulating GSH/GPX4 axis to inhibit ferroptosis
Abstract
Introduction: Ferroptosis, induced by iron overload and an imbalance in redox homeostasis, promotes the generation of reactive oxygen species (ROS), leading to iron-dependent lipid peroxides (LPO) and oxidative stress. Lipid peroxidation induced by reactive oxygen species is essential for the progression of spermatogenesis. However, its imbalance can lead to reproductive system damage and oligoasthenospermia, a critical cause of oligoasthenospermia. Isatin (ISA) is a naturally occurring compound that is widely distributed in lobsters, crustaceans, shellfish and various plants. It exhibits significant antioxidant and anti-aging properties, suggesting its potential as a therapeutic agent for the treatment of oligoasthenospermia. This study aimed to investigate the effects and mechanisms of ISA on oligoasthenospermia and to elucidate the underlying molecular pathways.
Methods: All mice were divided into normal group, model group and treatment group. Both model group and treatment group received a single intraperitoneal injection of 30 mg/kg BUS to create the model of oligoasthenospermia. After 2 weeks, the treatment group received different doses of 25, 50 and 100 mg/kg ISA by gavage for 28 days, and then mice were sacrificed and tested.
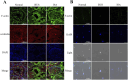
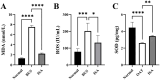
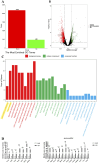
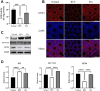
Results: The results demonstrated that ISA effectively reversed busulfan-induced reproductive system damage in mice. This included the restoration of testicular histomorphology, improvement in sperm concentration and motility, regulation of serum sex hormone levels, and normalization of various oxidative indices in testicular tissue. Furthermore, ISA successfully reversed testicular ferroptosis by restraining the translocation of nuclear factor erythroid 2-related factor 2 (NRF2) into the nucleus and improved oligoasthenospermia through the glutathione (GSH)/glutathione peroxidase 4 (GPX4) axis.
Discussion: ISA was found to effectively ameliorate oligoasthenospermia in mice, presenting a potential therapeutic option for patients with this condition.
Keywords: GSH/GPX4 axis; ferroptosis; isatin; oligoasthenospermia; spermatogenesis.
Copyright © 2024 Wang, Wang, Dong, Li, Ye, Zeng, Jiang, Shi, Wang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources

