AAVS1-targeted, stable expression of ChR2 in human brain organoids for consistent optogenetic control
- PMID: 39545087
- PMCID: PMC11558186
- DOI: 10.1002/btm2.10690
AAVS1-targeted, stable expression of ChR2 in human brain organoids for consistent optogenetic control
Abstract
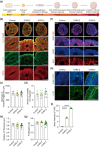
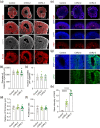
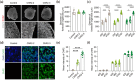
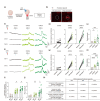
Self-organizing brain organoids provide a promising tool for studying human development and disease. Here we created human forebrain organoids with stable and homogeneous expression of channelrhodopsin-2 (ChR2) by generating AAVS1 safe harbor locus-targeted, ChR2 knocked-in human pluripotent stem cells (hPSCs), followed by the differentiation of these genetically engineered hPSCs into forebrain organoids. The resulting ChR2-expressing human forebrain organoids showed homogeneous cellular expression of ChR2 throughout entire regions without any structural and functional perturbations and displayed consistent and robust neural activation upon light stimulation, allowing for the non-virus mediated, spatiotemporal optogenetic control of neural activities. Furthermore, in the hybrid platform in which brain organoids are connected with spinal cord organoids and skeletal muscle spheroids, ChR2 knocked-in forebrain organoids induced strong and consistent muscle contraction upon brain-specific optogenetic stimulation. Our study thus provides a novel, non-virus mediated, preclinical human organoid system for light-inducible, consistent control of neural activities to study neural circuits and dynamics in normal and disease-specific human brains as well as neural connections between brain and other peripheral tissues.
Keywords: AAVS1 locus; channelrhodopsin‐2; forebrain organoids; optogenetics.
© 2024 The Author(s). Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures


Similar articles
-
Light-stimulated insulin secretion from pancreatic islet-like organoids derived from human pluripotent stem cells.Mol Ther. 2023 May 3;31(5):1480-1495. doi: 10.1016/j.ymthe.2023.03.013. Epub 2023 Mar 16. Mol Ther. 2023. PMID: 36932674 Free PMC article.
-
Comparison of Nocifensive Behavior in NaV1.7-, NaV1.8-, and NaV1.9-Channelrhodopsin-2 Mice by Selective Optogenetic Activation of Targeted Sodium Channel Subtype-Expressing Afferents.J Neurosci Res. 2024 Oct;102(10):e25386. doi: 10.1002/jnr.25386. J Neurosci Res. 2024. PMID: 39364619
-
Optogenetically transduced human ES cell-derived neural progenitors and their neuronal progenies: Phenotypic characterization and responses to optical stimulation.PLoS One. 2019 Nov 11;14(11):e0224846. doi: 10.1371/journal.pone.0224846. eCollection 2019. PLoS One. 2019. PMID: 31710637 Free PMC article.
-
Optogenetics: Illuminating the Future of Hearing Restoration and Understanding Auditory Perception.Curr Gene Ther. 2024;24(3):208-216. doi: 10.2174/0115665232269742231213110937. Curr Gene Ther. 2024. PMID: 38676313 Review.
-
ChR2 transgenic animals in peripheral sensory system: Sensing light as various sensations.Life Sci. 2016 Apr 1;150:95-102. doi: 10.1016/j.lfs.2016.02.057. Epub 2016 Feb 21. Life Sci. 2016. PMID: 26903290 Review.
Cited by
-
Progress in spinal cord organoid research: advancing understanding of neural development, disease modelling, and regenerative medicine.Biomater Transl. 2024 Nov 15;5(4):355-371. doi: 10.12336/biomatertransl.2024.04.003. eCollection 2024. Biomater Transl. 2024. PMID: 39872925 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

