Nanostructured MnO x /g-C3N4 for photodegradation of sulfamethoxazole under visible light irradiation
- PMID: 39545170
- PMCID: PMC11561708
- DOI: 10.1039/d4ra05996d
Nanostructured MnO x /g-C3N4 for photodegradation of sulfamethoxazole under visible light irradiation
Abstract
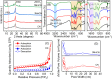
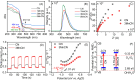
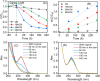
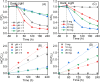
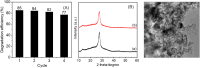
The effectiveness of g-C3N4 as photocatalyst is hindered by the rapid recombination of photo-generated electron/hole pairs. To improve its photocatalytic performance, the incorporation of g-C3N4 with co-catalysts can promote charge separation efficiency and enhance redox capabilities. In our study, a two-step approach involving calcination and solvothermal method was utilized to fabricate a proficient MnO x /g-C3N4 heterojunction photocatalyst with high photocatalytic activity. MnO x is effective at capturing holes to impede the recombination of electron/hole pairs. The MnO x /g-C3N4 composite shows a notable improvement in photocatalytic degradation of SMX, obtaining an 85% degradation rate, surpassing that of pure g-C3N4. Furthermore, the MnO x /g-C3N4 composite exhibits remarkable and enduring catalytic degradation capabilities for sulfamethoxazole (SMX), even after four consecutive reuse cycles. The intermediates produced in the MnO x /g-C3N4 system are found to be less hazardous to common aquatic creatures such as fish, daphnids, and green algae when compared to SMX. With its high tolerance, exceptional degradation ability, and minimal ecological risk, the MnO x /g-C3N4 composite emerges as a promising candidate for eliminating antibiotics from wastewater resources.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Synthesis of novel Type-II MnNb2O6/g-C3N4 Mott-Schottky heterojunction photocatalyst: Excellent photocatalytic performance and degradation mechanism of fluoroquinolone-based antibiotics.Chemosphere. 2023 Apr;321:138027. doi: 10.1016/j.chemosphere.2023.138027. Epub 2023 Jan 31. Chemosphere. 2023. PMID: 36736476
-
In-situ fabrication of Ag/P-g-C3N4 composites with enhanced photocatalytic activity for sulfamethoxazole degradation.J Hazard Mater. 2019 Mar 15;366:219-228. doi: 10.1016/j.jhazmat.2018.11.104. Epub 2018 Nov 29. J Hazard Mater. 2019. PMID: 30530013
-
Rational design direct Z-scheme BiOBr/g-C3N4 heterojunction with enhanced visible photocatalytic activity for organic pollutants elimination.RSC Adv. 2020 Jan 29;10(8):4681-4689. doi: 10.1039/c9ra10146b. eCollection 2020 Jan 24. RSC Adv. 2020. PMID: 35495249 Free PMC article.
-
Doped graphitic carbon nitride (g-C3N4) catalysts for efficient photodegradation of tetracycline antibiotics in aquatic environments.Environ Sci Pollut Res Int. 2023 Feb;30(10):24919-24926. doi: 10.1007/s11356-022-19766-y. Epub 2022 Mar 19. Environ Sci Pollut Res Int. 2023. PMID: 35306654 Review.
-
Graphitic carbon nitride based nanocomposites for the photocatalysis of organic contaminants under visible irradiation: Progress, limitations and future directions.Sci Total Environ. 2018 Aug 15;633:546-559. doi: 10.1016/j.scitotenv.2018.03.206. Epub 2018 Mar 28. Sci Total Environ. 2018. PMID: 29579666 Review.
References
LinkOut - more resources
Full Text Sources

