Research on a Minor Organism can also be Benefit the World: The Fascinating Cellular Slime Mold Dictyostelium discoideum
- PMID: 39545231
- PMCID: PMC11560335
- DOI: 10.14789/jmj.JMJ24-0021-R
Research on a Minor Organism can also be Benefit the World: The Fascinating Cellular Slime Mold Dictyostelium discoideum
Abstract
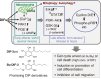
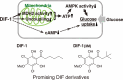
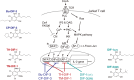
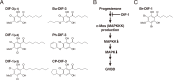
In 1985, when I entered the Graduate School of Science at Kyoto University, I began my research on cellular slime molds, a group of soil microorganisms. The cellular slime mold Dictyostelium discoideum is studied globally as a model organism for cell and developmental biology. I was conducting basic biological research into cell differentiation and migration using D. discoideum, and during this process, our research group made a discovery with potential implications for drug development. Specifically, we found that a chlorinated polyketide named differentiation-inducing factor 1 (DIF-1), derived from D. discoideum, exhibits antitumor activity. Based on this discovery, I began elucidating the mechanism of the antitumor action of DIF-1 and developing anticancer drugs using DIF-1 as a lead compound. During this period, in 1991, I obtained my Ph.D. in research related to D. discoideum cell differentiation, and subsequently served as a Japan Society for the Promotion of Science (JSPS) Special Research Fellow before joining the Institute for Molecular and Cellular Regulation (IMCR) at Gunma University in 1993. I then joined the Graduate School of Health and Sports Sciences at Juntendo University in 2015, where I have been until 2024. Throughout this period, I continued my research on DIF-1 and discovered that DIF-1 and its derivatives possess various biological activities ─ such as anti-diabetic, immunoregulatory, anti-bacterial, and anti-malarial activities ─ that could be applicable in drug development. In this review, I aim to present a segment of both our fundamental and applied research on D. discoideum and DIF-1.
Keywords: DIF-1; Dictyostelium discoideum; cellular slime mold; drug development.
© 2024 The Juntendo Medical Society.
Conflict of interest statement
The author has no conflict of interest to disclose.
Figures

Similar articles
-
Dictyostelium: An Important Source of Structural and Functional Diversity in Drug Discovery.Cells. 2018 Dec 21;8(1):6. doi: 10.3390/cells8010006. Cells. 2018. PMID: 30583484 Free PMC article. Review.
-
Biological Activities of Novel Derivatives of Differentiation-Inducing Factor 3 from Dictyostelium discoideum.Biol Pharm Bull. 2017;40(11):1941-1947. doi: 10.1248/bpb.b17-00484. Biol Pharm Bull. 2017. PMID: 29093342
-
Halogen-Substituted Derivatives of Dictyostelium Differentiation-Inducing Factor-1 Suppress Serum-Induced Cell Migration of Human Breast Cancer MDA-MB-231 Cells in Vitro.Biomolecules. 2019 Jun 28;9(7):256. doi: 10.3390/biom9070256. Biomolecules. 2019. PMID: 31261818 Free PMC article.
-
Antimicrobial Activities of Dictyostelium Differentiation-Inducing Factors and Their Derivatives.Biomolecules. 2019 Apr 27;9(5):163. doi: 10.3390/biom9050163. Biomolecules. 2019. PMID: 31035614 Free PMC article.
-
Diffusible signal molecules controlling cell differentiation and patterning in Dictyostelium.Dev Suppl. 1991;1:131-9. Dev Suppl. 1991. PMID: 1660326 Review.
Cited by
-
Biologically Active Compounds in True Slime Molds and Their Prospects for Sustainable Pest and Pathogen Control.Int J Mol Sci. 2025 Feb 24;26(5):1951. doi: 10.3390/ijms26051951. Int J Mol Sci. 2025. PMID: 40076575 Free PMC article. Review.
References
-
- Adl SM, Simpson AG, Farmer MA, et al. : The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J Eukaryot Microbiol, 2005; 52: 399-451. - PubMed
-
- Raper KB: Dictyostelium discoideum, A new species of slime mold from decaying forest leaves. J Agr Res, 1935; 55: 289-316.
-
- Newell PC: The development of the cellular slime mould Dictyostelium discoideum: a model system for the study of cellular differentiation. Essays Biochem, 1971; 7: 87-126. - PubMed

